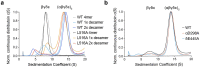A helical fulcrum in eIF2B coordinates allosteric regulation of stress signaling
- PMID: 37945896
- PMCID: PMC10972756
- DOI: 10.1038/s41589-023-01453-9
A helical fulcrum in eIF2B coordinates allosteric regulation of stress signaling
Abstract
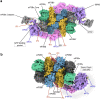
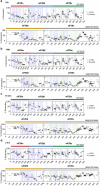
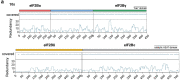
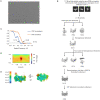
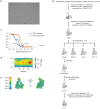
The integrated stress response (ISR) enables cells to survive a variety of acute stresses, but chronic activation of the ISR underlies age-related diseases. ISR signaling downregulates translation and activates expression of stress-responsive factors that promote return to homeostasis and is initiated by inhibition of the decameric guanine nucleotide exchange factor eIF2B. Conformational and assembly transitions regulate eIF2B activity, but the allosteric mechanisms controlling these dynamic transitions and mediating the therapeutic effects of the small-molecule ISR inhibitor ISRIB are unknown. Using hydrogen-deuterium exchange-mass spectrometry and cryo-electron microscopy, we identified a central α-helix whose orientation allosterically coordinates eIF2B conformation and assembly. Biochemical and cellular signaling assays show that this 'switch-helix' controls eIF2B activity and signaling. In sum, the switch-helix acts as a fulcrum of eIF2B conformational regulation and is a highly conserved actuator of ISR signal transduction. This work uncovers a conserved allosteric mechanism and unlocks new therapeutic possibilities for ISR-linked diseases.
© 2023. The Author(s).
Conflict of interest statement
P.W. is an inventor of ISRIB. A patent is held by the Regents of the University of California that describes ISRIB and its analogs. Rights to the invention have been licensed by UCSF to Calico. P.W. is a current employee and shareholder in Altos Labs, Inc. All other authors have no competing interests.
Figures