Checkpoint kinase 2 controls insulin secretion and glucose homeostasis
- PMID: 37945898
- PMCID: PMC11062908
- DOI: 10.1038/s41589-023-01466-4
Checkpoint kinase 2 controls insulin secretion and glucose homeostasis
Abstract
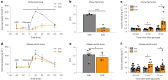
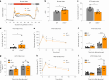
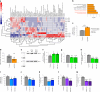
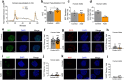
After the discovery of insulin, a century ago, extensive work has been done to unravel the molecular network regulating insulin secretion. Here we performed a chemical screen and identified AZD7762, a compound that potentiates glucose-stimulated insulin secretion (GSIS) of a human β cell line, healthy and type 2 diabetic (T2D) human islets and primary cynomolgus macaque islets. In vivo studies in diabetic mouse models and cynomolgus macaques demonstrated that AZD7762 enhances GSIS and improves glucose tolerance. Furthermore, genetic manipulation confirmed that ablation of CHEK2 in human β cells results in increased insulin secretion. Consistently, high-fat-diet-fed Chk2-/- mice show elevated insulin secretion and improved glucose clearance. Finally, untargeted metabolic profiling demonstrated the key role of the CHEK2-PP2A-PLK1-G6PD-PPP pathway in insulin secretion. This study successfully identifies a previously unknown insulin secretion regulating pathway that is conserved across rodents, cynomolgus macaques and human β cells in both healthy and T2D conditions.
© 2023. The Author(s).
Conflict of interest statement
S.C. is one of the cofounders of OncoBeat. S.C. is a consultant for Vesalius Therapeutics.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

