Adaptable, turn-on maturation (ATOM) fluorescent biosensors for multiplexed detection in cells
- PMID: 37945909
- PMCID: PMC11080272
- DOI: 10.1038/s41592-023-02065-w
Adaptable, turn-on maturation (ATOM) fluorescent biosensors for multiplexed detection in cells
Abstract
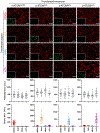
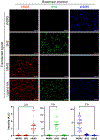
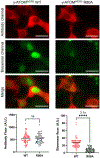
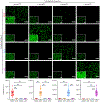
A grand challenge in biosensor design is to develop a single-molecule, fluorescent protein-based platform that can be easily adapted to recognize targets of choice. Here, we created a family of adaptable, turn-on maturation (ATOM) biosensors consisting of a monobody (circularly permuted at one of two positions) or a nanobody (circularly permuted at one of three positions) inserted into a fluorescent protein at one of three surface loops. Multiplexed imaging of live human cells coexpressing cyan, yellow and red ATOM sensors detected biosensor targets that were specifically localized to various subcellular compartments. Fluorescence activation involved ligand-dependent chromophore maturation with turn-on ratios of up to 62-fold in cells and 100-fold in vitro. Endoplasmic reticulum- and mitochondria-localized ATOM sensors detected ligands that were targeted to those organelles. The ATOM design was validated with three monobodies and one nanobody inserted into distinct fluorescent proteins, suggesting that customized ATOM sensors can be generated quickly.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures


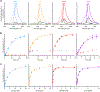
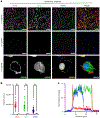
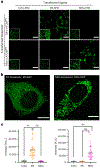
Update of
-
Adaptable, Turn-On Monobody (ATOM) Fluorescent Biosensors for Multiplexed Detection in Cells.bioRxiv [Preprint]. 2023 Mar 28:2023.03.28.534597. doi: 10.1101/2023.03.28.534597. bioRxiv. 2023. Update in: Nat Methods. 2023 Dec;20(12):1920-1929. doi: 10.1038/s41592-023-02065-w. PMID: 37034669 Free PMC article. Updated. Preprint.
References
-
- Nakai J, Ohkura M & Imoto K A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol 19, 137–141 (2001). - PubMed
-
- Nasu Y, Shen Y, Kramer L & Campbell RE Structure- and mechanism-guided design of single fluorescent protein-based biosensors. Nat. Chem. Biol 17, 509–518 (2021). - PubMed
-
- Inoue M. et al. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nat. Methods 12, 64–70 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

