Global variation in diabetes diagnosis and prevalence based on fasting glucose and hemoglobin A1c
- PMID: 37946056
- PMCID: PMC10667106
- DOI: 10.1038/s41591-023-02610-2
Global variation in diabetes diagnosis and prevalence based on fasting glucose and hemoglobin A1c
Abstract
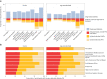
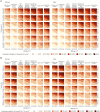
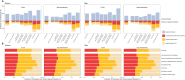
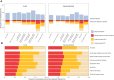
Fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) are both used to diagnose diabetes, but these measurements can identify different people as having diabetes. We used data from 117 population-based studies and quantified, in different world regions, the prevalence of diagnosed diabetes, and whether those who were previously undiagnosed and detected as having diabetes in survey screening, had elevated FPG, HbA1c or both. We developed prediction equations for estimating the probability that a person without previously diagnosed diabetes, and at a specific level of FPG, had elevated HbA1c, and vice versa. The age-standardized proportion of diabetes that was previously undiagnosed and detected in survey screening ranged from 30% in the high-income western region to 66% in south Asia. Among those with screen-detected diabetes with either test, the age-standardized proportion who had elevated levels of both FPG and HbA1c was 29-39% across regions; the remainder had discordant elevation of FPG or HbA1c. In most low- and middle-income regions, isolated elevated HbA1c was more common than isolated elevated FPG. In these regions, the use of FPG alone may delay diabetes diagnosis and underestimate diabetes prevalence. Our prediction equations help allocate finite resources for measuring HbA1c to reduce the global shortfall in diabetes diagnosis and surveillance.
© 2023. The Author(s).
Conflict of interest statement
A.N.W. reports an honorarium from Sanofi for serving as a panel member at an educational event on thyroid cancer. The authors are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Figures


References
MeSH terms
Substances
Grants and funding
- MC_UP_A620_1015/MRC_/Medical Research Council/United Kingdom
- P30 DK092926/DK/NIDDK NIH HHS/United States
- RG/19/4/34452/BHF_/British Heart Foundation/United Kingdom
- MC_PC_21003/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/2/MRC_/Medical Research Council/United Kingdom
- MC_U147585819/MRC_/Medical Research Council/United Kingdom
- MC_PC_21001/MRC_/Medical Research Council/United Kingdom
- MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/3/MRC_/Medical Research Council/United Kingdom
- 19583/VAC_/Versus Arthritis/United Kingdom
- MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_21022/MRC_/Medical Research Council/United Kingdom
- MC_U147585824/MRC_/Medical Research Council/United Kingdom
- MR/V034057/1/MRC_/Medical Research Council/United Kingdom
- MC_U147585827/MRC_/Medical Research Council/United Kingdom
- MC_PC_21000/MRC_/Medical Research Council/United Kingdom
- RG/13/16/30528/BHF_/British Heart Foundation/United Kingdom
- G0601019/MRC_/Medical Research Council/United Kingdom
- G0400491/MRC_/Medical Research Council/United Kingdom
- MC_UP_A620_1016/MRC_/Medical Research Council/United Kingdom

