FUT3 facilitates glucose metabolism of lung adenocarcinoma via activation of NF-κB pathway
- PMID: 37946130
- PMCID: PMC10636925
- DOI: 10.1186/s12890-023-02688-x
FUT3 facilitates glucose metabolism of lung adenocarcinoma via activation of NF-κB pathway
Abstract
Objective: Fucosyltransferases (FUTs) molecules have been identified to be involved in carcinogenesis of malignant tumors. Nevertheless, the biological function of fucosyltransferases-3 (FUT3) in lung adenocarcinoma (LUAD) malignant phenotype remains unclear. Herein, we investigated the association between FUT3 and LUAD pathological process.
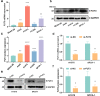
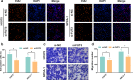
Methods: Immunochemistry, RT-qPCR and western blot assays were conducted to evaluate the expression of FUT3 in LUAD and corresponding adjacent tissues. The prognostic value of FUT3 was assessed via Kaplan‑Meier plotter database. The biological process and potential mechanism of FUT3 in LUAD were conducted via GSEA. Additionally, immunofluorescence and metabolite activity detection were performed to determine the potential role of FUT3 in LUAD glucose metabolism. The active biomarkers associated with NF-κB signaling pathway were detected via western blot. Subcutaneous tumor model was conducted to analyze the effect of FUT3 on tumorigenesis of LUAD.
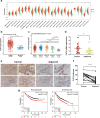
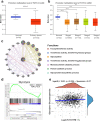
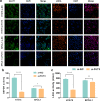
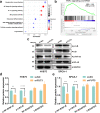
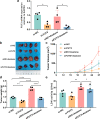
Results: FUT3 was remarkably upregulated in LUAD tissues compared with adjacent tissues from individuals. FUT3 overexpression may predict poor prognosis of LUAD patients. Knockdown of FUT3 significantly inhibited tumor proliferation, migration and glucometabolic alteration in LUAD cells. Moreover, GSEA demonstrated that elevated FUT3 was positively related to NF-κB signaling pathway. Additionally, in vitro and in vivo assays also indicated that downregulation of FUT3 resulted in the suppression of oncogenesis and glucose metabolism via inactivation of NF-κB pathway.
Conclusion: Our findings demonstrated that FUT3 was involved in glucometabolic process and tumorigenesis of LUAD via NF-κB signaling pathway. FUT3 may be an optimal target for diagnosis and treatment of LUAD patients.
Keywords: FUT3; Glucose metabolism; Lung adenocarcinoma; NF-κB pathway.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Transmembrane p24 trafficking protein 2 regulates inflammation through the TLR4/NF-κB signaling pathway in lung adenocarcinoma.World J Surg Oncol. 2022 Feb 8;20(1):32. doi: 10.1186/s12957-021-02477-y. World J Surg Oncol. 2022. PMID: 35135563 Free PMC article.
-
FAM83B promotes the invasion of primary lung adenocarcinoma via PI3K/AKT/NF-κB pathway.BMC Pulm Med. 2023 Jan 23;23(1):32. doi: 10.1186/s12890-022-02303-5. BMC Pulm Med. 2023. PMID: 36690987 Free PMC article.
-
Investigating the functional role of SETD6 in lung adenocarcinoma.BMC Cancer. 2023 Jan 6;23(1):18. doi: 10.1186/s12885-022-10476-9. BMC Cancer. 2023. PMID: 36604642 Free PMC article.
-
RTKN2 Inhibits the Growth, Migration, Invasion and Glycolysis of Lung Adenocarcinoma Cells by Inactivating the NF-κB Signalling Pathway.Biochem Genet. 2023 Oct;61(5):2135-2148. doi: 10.1007/s10528-023-10352-6. Epub 2023 Mar 23. Biochem Genet. 2023. PMID: 36952123 Free PMC article.
-
The upregulation of TMPRSS4, partly ascribed to the downregulation of miR‑125a‑5p, promotes the growth of human lung adenocarcinoma via the NF‑κB signaling pathway.Int J Oncol. 2018 Jul;53(1):148-158. doi: 10.3892/ijo.2018.4396. Epub 2018 May 7. Int J Oncol. 2018. PMID: 29750426 Free PMC article.
Cited by
-
The key role of the NUDT3 gene in lung adenocarcinoma progression and its association with the manganese ion metabolism family.Discov Oncol. 2025 Jan 29;16(1):98. doi: 10.1007/s12672-025-01844-5. Discov Oncol. 2025. PMID: 39878845 Free PMC article.
-
N-glycans in lung tissue specimens: a prospective target for enhanced cancer diagnosis and prognosis.J Transl Med. 2025 Aug 14;23(1):918. doi: 10.1186/s12967-025-06904-6. J Transl Med. 2025. PMID: 40813986 Free PMC article.
-
Genome-wide association studies in a large Korean cohort identify quantitative trait loci for 36 traits and illuminate their genetic architectures.Nat Commun. 2025 May 28;16(1):4935. doi: 10.1038/s41467-025-59950-5. Nat Commun. 2025. PMID: 40436827 Free PMC article.
-
Genome-wide association studies in a large Korean cohort identify novel quantitative trait loci for 36 traits and illuminate their genetic architectures.medRxiv [Preprint]. 2025 Jan 7:2024.05.17.24307550. doi: 10.1101/2024.05.17.24307550. medRxiv. 2025. Update in: Nat Commun. 2025 May 28;16(1):4935. doi: 10.1038/s41467-025-59950-5. PMID: 38798434 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

