A single-center observational study on long-term neurodevelopmental outcomes in children with tuberous sclerosis complex
- PMID: 37946245
- PMCID: PMC10637019
- DOI: 10.1186/s13023-023-02959-0
A single-center observational study on long-term neurodevelopmental outcomes in children with tuberous sclerosis complex
Abstract
Background: Tuberous sclerosis complex (TSC) is a rare multisystem disorder caused by mutations in the TSC1 or TSC2 gene. More than 90% of patients with TSC develop neurological and/or neuropsychiatric manifestations. The aim of the present study was to determine the developmental and cognitive long-term outcomes of pediatric TSC patients.
Methods: This cross-sectional, monocenter study included pediatric TSC patients who received multidisciplinary long-term care with a last visit between 2005 and 2019. Neurological manifestations and cognitive development (BSID, K-ABC) were analyzed in relation to age and type of mutation.
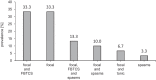
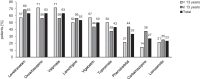
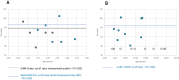
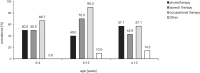
Results: Thirty-five patients aged 13.5 ± 7.8 years were included in the study. Diagnosis was confirmed genetically in 65.7% of patients (TSC1, 26.1%; TSC2, 65.2%; NMI, 8.7%). Mean age at diagnosis was 1.3 ± 3.5 years; 74.3% of the patients had been diagnosed within the first year of life due to seizures (62.9%) or/and cardiac rhabdomyomas (28.6%). The most common TSC manifestations included structural brain lesions (cortical tubers, 91.4%; subependymal nodules, 82.9%), epilepsy (85.7%), and cardiac rhabdomyomas (62.9%). Mean age at seizure onset was 1.5 ± 2.3 years, with onset in 80.0% of patients within the first two years of life. Infantile spasms, which were the first seizure type in 23.3% of the patients, developed earlier (0.6 ± 0.4 years) than focal seizures (1.8 ± 2.5 years). Refractory epilepsy was present in 21 (70.0%) patients, mild or severe intellectual impairment in 66.6%, and autism spectrum disorders in 11.4%. Severe cognitive impairment (33.3%) was significantly associated with epilepsy type and age at seizure onset (p < 0.05).
Conclusions: The results emphasized the phenotypic variability of pediatric-onset TSC and the high rate of neurological and neuropsychiatric morbidity. Early-onset refractory epilepsy was associated with impaired cognitive development. Children of all ages with TSC require multidisciplinary long-term care and individual early-intervention programs.
Keywords: Antiseizure medication; Autism; Cognition; Rare disease; Refractory epilepsy; Tuberous sclerosis complex.
© 2023. The Author(s).
Conflict of interest statement
RT received honoraria and personal lectures fees from Eisai, Desitin, Roche, Sanofi, and Biogen. HH has served on the scientific advisory board of Angelini, Corlieve, Eisai, Neuroventis, GW/Jazz, Sandoz, UCB Pharma, UNEEG and Zogenix. He served on the speakers’ bureau of or received unrestricted grants from Ad-Tech, Alnylam, Angelini, Bracco, Desitin, Eisai, GW, Micromed, Nihon Kohden, Novartis, Pfizer, and UCB Pharma. SG, DM, JV, MH, SS, AC, and AKW report no conflict of interest.
Figures
References
-
- De Vries PJ, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. TOSCA consortium and TOSCA investigators. TSC-associated neuropsychiatric disorders (TAND): findings from the TOSCA natural history study. Orphanet J Rare Dis. 2018;13:157. doi: 10.1186/s13023-018-0901-8. - DOI - PMC - PubMed
-
- Davis PE, Filip-Dhima R, Sideridis G, Peters JM, Au KS, Peters JM, et al. Tuberous sclerosis complex autism center of excellence research network. 2017 Presentation and diagnosis of tuberous sclerosis complex in infants. Pediatrics. 2017;140:e20164040. doi: 10.1542/peds.2016-4040. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

