A prospective multi-institutional study of eculizumab to treat high-risk stem cell transplantation-associated TMA
- PMID: 37946262
- PMCID: PMC10972707
- DOI: 10.1182/blood.2023022526
A prospective multi-institutional study of eculizumab to treat high-risk stem cell transplantation-associated TMA
Abstract
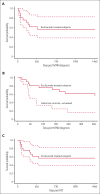
High-risk, complement mediated, untreated transplant-associated thrombotic microangiopathy (hrTMA) has dismal outcomes due to multi-organ dysfunction syndrome (MODS). The complement C5 blocker eculizumab shows promising results in hrTMA, but has not been prospectively studied in hematopoietic stem cell transplant (HCT) recipients. We performed the first multi-institutional prospective study in children and young adults to evaluate eculizumab as an early targeted intervention for hrTMA/MODS. We hypothesized that eculizumab would more than double survival in HCT recipients with hrTMA, compared to our prior study of prospectively screened, untreated hrTMAs serving as historical controls. HrTMA features (elevated terminal complement (sC5b-9) and proteinuria measured by random urine protein/creatinine ratio (≥1mg/mg)) were required for inclusion. The primary endpoint was survival at 6 six-months from hrTMA diagnosis. Secondary endpoints were cumulative incidence of MODS 6 months after hrTMA diagnosis and 1-year posttransplant survival. Eculizumab dosing included intensive loading, induction, and maintenance phases for up to 24 weeks of therapy. All 21 evaluated study subjects had MODS. Primary and secondary study endpoints were met by demonstrating survival of 71% (P < .0001) 6 months after hrTMA diagnosis and 62% 1 year after transplant. Of fifteen survivors, 11 (73%) fully recovered organ function and are well. Our study demonstrates significant improvement in survival and recovery of organ function in hrTMA using an intensified eculizumab dosing and real time biomarker monitoring. This study serves as a benchmark for planning future studies that should focus on preventative measures or targeted therapy to be initiated prior to organ injury. This trial was registered at www.clinicaltrials.gov as #NCT03518203.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: S.J. and B.L.L. are coinventors on US patent number US 10,815,296 B2. S.J. is a lead PI for the National Institute of Health–funded multi-institutional study investigating TA-TMA (R01HD093773), and received honoraria for lectures and consultancy from Omeros, Sobi, and Alexion Pharmaceuticals. S.M.D. has received research support from Alexion Pharmaceuticals, and consultants with AlloVir. C.E.D. has received honoraria from Omeros. A.S. received honoraria from Sobi. A.T.-C. received honoraria from Jazz Pharmaceuticals. K.M. and S.J. are listed as inventors in pending patent applications. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Averting a terminal transplant cascade.Blood. 2024 Mar 21;143(12):1059-1060. doi: 10.1182/blood.2023023199. Blood. 2024. PMID: 38512264 No abstract available.
References
-
- Schoettler M, Carreras E, Cho B, et al. Harmonizing definitions for diagnostic criteria and prognostic assessment of transplantation-associated thrombotic microangiopathy: a report on behalf of the European Society for Blood and Marrow Transplantation, American Society for Transplantation and Cellular Therapy, Asia-Pacific Blood and Marrow Transplantation Group, and Center for International Blood and Marrow Transplant Research. Transplant Cell Ther. 2023;29(3):151–163. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

