Moving toward a contemporary classification of drug-induced kidney disease
- PMID: 37946280
- PMCID: PMC10633929
- DOI: 10.1186/s13054-023-04720-2
Moving toward a contemporary classification of drug-induced kidney disease
Abstract
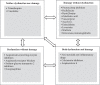
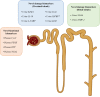
Drug-induced kidney disease (DIKD) accounts for about one-fourth of all cases of acute kidney injury (AKI) in hospitalized patients, especially in critically ill setting. There is no standard definition or classification system of DIKD. To address this, a phenotype definition of DIKD using expert consensus was introduced in 2015. Recently, a novel framework for DIKD classification was proposed that incorporated functional change and tissue damage biomarkers. Medications were stratified into four categories, including "dysfunction without damage," "damage without dysfunction," "both dysfunction and damage," and "neither dysfunction nor damage" using this novel framework along with predominant mechanism(s) of nephrotoxicity for drugs and drug classes. Here, we briefly describe mechanisms and provide examples of drugs/drug classes related to the categories in the proposed framework. In addition, the possible movement of a patient's kidney disease between certain categories in specific conditions is considered. Finally, opportunities and barriers to adoption of this framework for DIKD classification in real clinical practice are discussed. This new classification system allows congruencies for DIKD with the proposed categorization of AKI, offering clarity as well as consistency for clinicians and researchers.
Keywords: Acute kidney injury; Adverse drug event; Critical care; Critically ill; Drug-related side effects and adverse reactions; Intensive care units.
© 2023. The Author(s).
Conflict of interest statement
MO received speaker honoraria from Fresenius Medical, Baxter, Gilead, and BioMerieux, and research funding from Fresenius Medical, Baxter, La Jolla Pharma, and BioMerieux. JAK has received grant support and consulting fees from BioMerieux and is a full-time employee of Spectral Medical. PTM has received consulting fees from AM-Pharma, Renibus Therapeutics, and Novartis. KK has received research grants from Philips Research North America and Google; Speaker honorarium: Nikkiso Critical Care Medical Supplies (Shanghai) Co., Ltd; Funding: National Institute of Diabetes and Digestive and Kidney Diseases grant (R01DK131586), Baxter, La Jolla Pharma, and BioMerieux. Conflict of Interest Disclosures: National Institute of Diabetes and Digestive and Kidney Diseases grants and consulting fees to Mayo Clinic from Baxter Inc. LA has received research funding from Sony Electronics, and honoraria/travel support from the American Board of Internal Medicine. SLG receives consulting fees from Baxter, Medtronic, NuWellis, SeaStar Medical, ExThera, BioPorto Diagnostics, Leadiant, Alexion, Acclerex, and Portero. He receives grant funding from Baxter, BioPorto Diagnostics, NuWellis, SeaStar Medical, and ExThera. He receives speaking fees from Baxter, BioPorto Diagnostics, Fresenius, and NuWellis. He has received stock options from MediBeacon. He receives royalties from RAIDAR Health and Vigilanz. AB received research funding from the National Institutes of Health and Astute Medical. AB reports Method and Apparatus for Pervasive Patient Monitoring, US Patent Number 11424028B2, date of patent August 23, 2022; Systems and Methods for Providing an Acuity Score for Critically Ill or Injured Patients, US Patent Application Publication 20220044809A1, publication date February 10, 2022; and Method and Apparatus for Prediction of Complications after Surgery, US Patent Application Publication Number 20200161000A1, publication date May 21, 2020. SKG receives grant funding from the National Institute of Diabetes and Digestive and Kidney Diseases R01DK121730 and U01DK130010, the National Center for Complementary and Integrative Health U54AT008909 and the Jewish Healthcare Foundation.MO received speaker honoraria from Fresenius Medical, Baxter, Gilead, and BioMerieux, and research funding from Fresenius Medical, Baxter, La Jolla Pharma, and BioMerieux. JAK has received grant support and consulting fees from BioMerieux and is a full-time employee of Spectral Medical. PTM has received consulting fees from AM-Pharma, Renibus Therapeutics, and Novartis. KK has received research grants from Philips Research North America and Google; Speaker honorarium: Nikkiso Critical Care Medical Supplies (Shanghai) Co., Ltd; Funding: National Institute of Diabetes and Digestive and Kidney Diseases grant (R01DK131586), Baxter, La Jolla Pharma, and BioMerieux. Conflict of Interest Disclosures: National Institute of Diabetes and Digestive and Kidney Diseases grants and consulting fees to Mayo Clinic from Baxter Inc. LA has received research funding from Sony Electronics, and honoraria/travel support from the American Board of Internal Medicine. SLG receives consulting fees from Baxter, Medtronic, NuWellis, SeaStar Medical, ExThera, BioPorto Diagnostics, Leadiant, Alexion, Acclerex, and Portero. He receives grant funding from Baxter, BioPorto Diagnostics, NuWellis, SeaStar Medical, and ExThera. He receives speaking fees from Baxter, BioPorto Diagnostics, Fresenius, and NuWellis. He has received stock options from MediBeacon. He receives royalties from RAIDAR Health and Vigilanz. AB received research funding from the National Institutes of Health and Astute Medical. AB reports Method and Apparatus for Pervasive Patient Monitoring, US Patent Number 11424028B2, date of patent August 23, 2022; Systems and Methods for Providing an Acuity Score for Critically Ill or Injured Patients, US Patent Application Publication 20220044809A1, publication date February 10, 2022; and Method and Apparatus for Prediction of Complications after Surgery, US Patent Application Publication Number 20200161000A1, publication date May 21, 2020. SKG receives grant funding from the National Institute of Diabetes and Digestive and Kidney Diseases R01DK121730 and U01DK130010, the National Center for Complementary and Integrative Health U54AT008909 and the Jewish Healthcare Foundation.SKG holds an executive position in the Society of Critical Care Medicine. The content of this manuscript is solely the responsibility of the author and does not represent the official views of the Society of Critical Care Medicine.
Figures
References
-
- Endre ZH, Kellum JA, Di Somma S, Doi K, Goldstein SL, Koyner JL, et al. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth acute dialysis quality initiative consensus conference. Contrib Nephrol. 2013;182:30–44. doi: 10.1159/000349964. - DOI - PubMed
-
- Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw Open. 2020;3(10):e2019209. doi: 10.1001/jamanetworkopen.2020.19209. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

