Biallelic CRELD1 variants cause a multisystem syndrome, including neurodevelopmental phenotypes, cardiac dysrhythmias, and frequent infections
- PMID: 37947183
- PMCID: PMC10932913
- DOI: 10.1016/j.gim.2023.101023
Biallelic CRELD1 variants cause a multisystem syndrome, including neurodevelopmental phenotypes, cardiac dysrhythmias, and frequent infections
Abstract
Purpose: We sought to delineate a multisystem disorder caused by recessive cysteine-rich with epidermal growth factor-like domains 1 (CRELD1) gene variants.
Methods: The impact of CRELD1 variants was characterized through an international collaboration utilizing next-generation DNA sequencing, gene knockdown, and protein overexpression in Xenopus tropicalis, and in vitro analysis of patient immune cells.
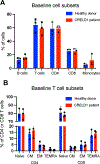
Results: Biallelic variants in CRELD1 were found in 18 participants from 14 families. Affected individuals displayed an array of phenotypes involving developmental delay, early-onset epilepsy, and hypotonia, with about half demonstrating cardiac arrhythmias and some experiencing recurrent infections. Most harbored a frameshift in trans with a missense allele, with 1 recurrent variant, p.(Cys192Tyr), identified in 10 families. X tropicalis tadpoles with creld1 knockdown displayed developmental defects along with increased susceptibility to induced seizures compared with controls. Additionally, human CRELD1 harboring missense variants from affected individuals had reduced protein function, indicated by a diminished ability to induce craniofacial defects when overexpressed in X tropicalis. Finally, baseline analyses of peripheral blood mononuclear cells showed similar proportions of immune cell subtypes in patients compared with healthy donors.
Conclusion: This patient cohort, combined with experimental data, provide evidence of a multisystem clinical syndrome mediated by recessive variants in CRELD1.
Keywords: CRELD1; Cardiac arrythmia; Developmental delay; Epilepsy; Hypotonia.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Two authors report part ownership of startup companies unrelated to this work: Qiyas Higher Health (Saquib A. Lakhani) and Victory Genomics (Saquib A. Lakhani and Mustafa K. Khokha). Kirsty McWalter is an employee of GeneDx. Kimberly Nugent is currently an employee of Cooper Surgical. Bryan Mak is currently an employee of Genome Medical. All other authors declare no conflicts of interest.
Figures



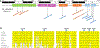

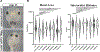
References
-
- Rupp PA, Fouad GT, Egelston CA, et al. Identification, genomic organization and mRNA expression of CRELD1, the founding member of a unique family of matricellular proteins. Gene. 2002;293(1–2):47–57. - PubMed
-
- Bonaguro L, Kohne M, Schmidleithner L, et al. CRELD1 modulates homeostasis of the immune system in mice and humans. Nat Immunol. 2020;21(12):1517–1527. - PubMed
-
- Mass E, Wachten D, Aschenbrenner AC, Voelzmann A, Hoch M. Murine Creld1 controls cardiac development through activation of calcineurin/NFATc1 signaling. Dev Cell. 2014;28(6):711–726. - PubMed
-
- Tombling BJ, Wang CK, Craik DJ. EGF-like and Other Disulfide-rich Microdomains as Therapeutic Scaffolds. Angew Chem Int Ed Engl. 2020;59(28):11218–11232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 NS108874/NS/NINDS NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- R01 NS131512/NS/NINDS NIH HHS/United States
- MC_EX_MR/M009203/1/MRC_/Medical Research Council/United Kingdom
- ImNIH/Intramural NIH HHS/United States
- U54 HD086984/HD/NICHD NIH HHS/United States
- U24 NS120854/NS/NINDS NIH HHS/United States
- K02 NS112600/NS/NINDS NIH HHS/United States
- R01 NS118522/NS/NINDS NIH HHS/United States
- UM1 HG008900/HG/NHGRI NIH HHS/United States
- R01 HG009141/HG/NHGRI NIH HHS/United States
- MC_PC_14089/MRC_/Medical Research Council/United Kingdom
- MR/M009203/1/MRC_/Medical Research Council/United Kingdom
- U01 NS134356/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 HD102186/HD/NICHD NIH HHS/United States
- S10 OD030363/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

