Generation of Fibrotic Liver Organoids Using Hepatocytes, Primary Liver Sinusoidal Endothelial Cells, Hepatic Stellate Cells, and Macrophages
- PMID: 37947592
- PMCID: PMC10647544
- DOI: 10.3390/cells12212514
Generation of Fibrotic Liver Organoids Using Hepatocytes, Primary Liver Sinusoidal Endothelial Cells, Hepatic Stellate Cells, and Macrophages
Abstract
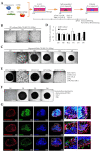
Liver organoids generated with single or multiple cell types have been used to investigate liver fibrosis development, toxicity, pathogenesis, and drug screening. However, organoid generation is limited by the availability of cells isolated from primary tissues or differentiated from various stem cells. To ensure cell availability for organoid formation, we investigated whether liver organoids could be generated with cell-line-based Huh-7 hepatocellular carcinoma cells, macrophages differentiated from THP-1 monocytes, and LX-2 hepatic stellate cells (HSCs) and primary liver sinusoidal endothelial cells (LSECs). In liver organoids, hepatocyte-, LSEC-, macrophage-, and HSC-related gene expression increased relative to that in two-dimensional (2D)-cultured Huh-7/LSEC/THP-1/LX-2 cells without Matrigel. Thioacetamide (TAA) increased α-smooth muscle actin expression in liver organoids but not in 2D-cultured cells, whereas in TAA-treated organoids, the expression of hepatic and LSEC markers decreased and that of macrophage and HSC markers increased. TAA-induced fibrosis was suppressed by treatment with N-acetyl-L-cysteine or tumor-necrosis-factor-stimulated gene 6 protein. The results showed that liver toxicants could induce fibrotic and inflammatory responses in liver organoids comprising Huh-7/LSEC/macrophages/LX-2 cells, resulting in fibrotic liver organoids. We propose that cell-line-based organoids can be used for disease modeling and drug screening to improve liver fibrosis treatment.
Keywords: Matrigel; fibrosis; inflammation; liver organoid; three-dimensional culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

