Treating Parkinson's Disease with Human Bone Marrow Mesenchymal Stem Cell Secretome: A Translational Investigation Using Human Brain Organoids and Different Routes of In Vivo Administration
- PMID: 37947643
- PMCID: PMC10650433
- DOI: 10.3390/cells12212565
Treating Parkinson's Disease with Human Bone Marrow Mesenchymal Stem Cell Secretome: A Translational Investigation Using Human Brain Organoids and Different Routes of In Vivo Administration
Abstract
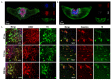
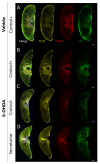
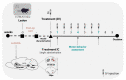
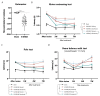
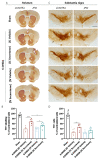
Parkinson's disease (PD) is the most common movement disorder, characterized by the progressive loss of dopaminergic neurons from the nigrostriatal system. Currently, there is no treatment that retards disease progression or reverses damage prior to the time of clinical diagnosis. Mesenchymal stem cells (MSCs) are one of the most extensively studied cell sources for regenerative medicine applications, particularly due to the release of soluble factors and vesicles, known as secretome. The main goal of this work was to address the therapeutic potential of the secretome collected from bone-marrow-derived MSCs (BM-MSCs) using different models of the disease. Firstly, we took advantage of an optimized human midbrain-specific organoid system to model PD in vitro using a neurotoxin-induced model through 6-hydroxydopamine (6-OHDA) exposure. In vivo, we evaluated the effects of BM-MSC secretome comparing two different routes of secretome administration: intracerebral injections (a two-site single administration) against multiple systemic administration. The secretome of BM-MSCs was able to protect from dopaminergic neuronal loss, these effects being more evident in vivo. The BM-MSC secretome led to motor function recovery and dopaminergic loss protection; however, multiple systemic administrations resulted in larger therapeutic effects, making this result extremely relevant for potential future clinical applications.
Keywords: Parkinson’s disease; brain organoids; mesenchymal stem cells; neurodegeneration; pre-clinical study; stem cell secretome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zahoor I., Shafi A., Haq E. Parkinson’s Disease: Pathogenesis and Clinical Aspects. Volume 311. Codon Publications; Singapore: 2018. Pharmacological Treatment of Parkinson’s Disease; pp. 129–144.
Publication types
MeSH terms
Substances
Grants and funding
- UIDB/50026/2020/Fundação para a Ciência e Tecnologia
- UIDP/50026/2020/Fundação para a Ciência e Tecnologia
- NORTE-01-0145-FEDER-000039/Norte Portugal Regional Operational Programme
- LCF/PR/HP20/52300001./Iniciativa Ibérica de Investigación y Innovación Biomedica
- (FNR/NCER13/BM/11264123)/Luxembourg National Research Fund
LinkOut - more resources
Full Text Sources
Medical

