Biocompatibility Evaluation of TiO2, Fe3O4, and TiO2/Fe3O4 Nanomaterials: Insights into Potential Toxic Effects in Erythrocytes and HepG2 Cells
- PMID: 37947670
- PMCID: PMC10648038
- DOI: 10.3390/nano13212824
Biocompatibility Evaluation of TiO2, Fe3O4, and TiO2/Fe3O4 Nanomaterials: Insights into Potential Toxic Effects in Erythrocytes and HepG2 Cells
Abstract
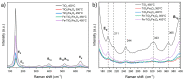
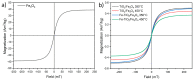
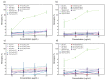
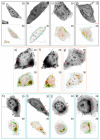
Nanomaterials such as titanium dioxide and magnetite are increasingly used in several fields, such as water remediation and agriculture. However, this has raised environmental concerns due to potential exposure to organisms like humans. Nanomaterials can cause adverse interactions depending on physicochemical characteristics, like size, morphology, and composition, when interacting with living beings. To ensure safe use and prevent the risk of exposure to nanomaterials, their biocompatibility must be assessed. In vitro cell cultures are beneficial for assessing nanomaterial-cell interactions due to their easy handling. The present study evaluated the biocompatibility of TiO2, Fe3O4, and TiO2/Fe3O4 nanomaterials thermally treated at 350 °C and 450 °C in erythrocytes and HepG2 cells. According to the hemolysis experiments, non-thermally treated NMs are toxic (>5% hemolysis), but their thermally treated counterparts do not present toxicity (<2%). This behavior indicates that the toxicity derives from some precursor (solvent or surfactant) used in the synthesis of the nanomaterials. All the thermally treated nanomaterials did not show hemolytic activity under different conditions, such as low-light exposure or the absence of blood plasma proteins. In contrast, non-thermally treated nanomaterials showed a high hemolytic behavior, which was reduced after the purification (washing and thermal treatment) of nanomaterials, indicating the presence of surfactant residue used during synthesis. An MTS cell viability assay shows that calcined nanomaterials do not reduce cell viability (>11%) during 24 h of exposure. On the other hand, a lactate dehydrogenase leakage assay resulted in a higher variability, indicating that several nanomaterials did not cause an increase in cell death as compared to the control. However, a holotomographic microscopy analysis reveals a high accumulation of nanomaterials in the cell structure at a low concentration (10 µg mL-1), altering cell morphology, which could lead to cell membrane damage and cell viability reduction.
Keywords: cell viability; hemolysis assay; holotomography; nanomaterials; nanotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Nasrollahzadeh M., Sajadi S.M., Sajjadi M., Issaabadi Z. Chapter 1—An Introduction to Nanotechnology. In: Nasrollahzadeh M., Sajadi S.M., Sajjadi M., Issaabadi Z., Atarod M., editors. Interface Science and Technology. Volume 28. Elsevier; Amsterdam, The Netherlands: 2019. pp. 1–27.
-
- Sardoiwala M.N., Kaundal B., Choudhury S.R. Toxic Impact of Nanomaterials on Microbes, Plants and Animals. Environ. Chem. Lett. 2018;16:147–160. doi: 10.1007/s10311-017-0672-9. - DOI
-
- Rind I.K., Tuzen M., Sarı A., Lanjwani M.F., Memon N., Saleh T.A. Synthesis of TiO2 Nanoparticles Loaded on Magnetite Nanoparticles Modified Kaolinite Clay (KC) and Their Efficiency for As(III) Adsorption. Chem. Eng. Res. Des. 2023;191:523–536. doi: 10.1016/j.cherd.2023.01.046. - DOI
LinkOut - more resources
Full Text Sources

