Comparing the Efficacy and Safety of Galcanezumab Versus Rimegepant for Prevention of Episodic Migraine: Results from a Randomized, Controlled Clinical Trial
- PMID: 37948006
- PMCID: PMC10787669
- DOI: 10.1007/s40120-023-00562-w
Comparing the Efficacy and Safety of Galcanezumab Versus Rimegepant for Prevention of Episodic Migraine: Results from a Randomized, Controlled Clinical Trial
Abstract
Introduction: There have been no prior trials directly comparing the efficacy of different calcitonin gene-related peptide (CGRP) antagonists for migraine prevention. Reported are the results from the first head-to-head study of two CGRP antagonists, galcanezumab (monoclonal antibody) versus rimegepant (gepant), for the prevention of episodic migraine.
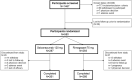
Methods: In this 3-month, double-blind, double-dummy study, participants were randomized (1:1) to subcutaneous (SC) galcanezumab 120 mg per month (after a 240 mg loading dose) and a placebo oral disintegrating tablet (ODT) every other day (q.o.d.) or to rimegepant 75 mg ODT q.o.d. and a monthly SC placebo. The primary endpoint was the proportion of participants with a ≥ 50% reduction in migraine headache days per month from baseline across the 3-month double-blind treatment period. Key secondary endpoints were overall mean change from baseline in: migraine headache days per month across 3 months and at month 3, 2, and 1; migraine headache days per month with acute migraine medication use; Migraine-Specific Quality of Life Questionnaire Role Function-Restrictive domain score at month 3; and a ≥ 75% and 100% reduction from baseline in migraine headache days per month across 3 months.
Results: Of 580 randomized participants (galcanezumab: 287, rimegepant: 293; mean age: 42 years), 83% were female and 81% Caucasian. Galcanezumab was not superior to rimegepant in achieving a ≥ 50% reduction from baseline in migraine headache days per month (62% versus 61% respectively; P = 0.70). Given the pre-specified multiple testing procedure, key secondary endpoints cannot be considered statistically significant. Overall, treatment-emergent adverse events were reported by 21% of participants, with no significant differences between study intervention groups.
Conclusions: Galcanezumab was not superior to rimegepant for the primary endpoint; however, both interventions demonstrated efficacy as preventive treatments in participants with episodic migraine. The efficacy and safety profiles observed in galcanezumab-treated participants were consistent with previous studies.
Trial registration: ClinTrials.gov-NCT05127486 (I5Q-MC-CGBD).
Keywords: CGRP antagonist; Calcitonin gene-related peptide (CGRP); Clinical study; Comparative efficacy; Galcanezumab; Gepant; Head-to-head; Migraine; Prevention; Rimegepant.
Plain language summary
Galcanezumab and rimegepant are preventive treatments for episodic migraine. The goal of this study was to compare the efficacy of galcanezumab and rimegepant in reducing the number of monthly migraine headaches and to determine if galcanezumab was better than rimegepant. The study provides important information to doctors and their patients when making treatment decisions.People with episodic migraine were assigned to the galcanezumab (given as an injection under the skin) or rimegepant (given as a tablet that dissolves in the mouth) group and treated for 3 months. The doctor and the patient did not know which group they were assigned to, and to keep it unknown to both, people in the galcanezumab group got an injection with real medicine and a fake tablet, and people in the rimegepant group got a tablet with real medicine and a fake injection. The researchers wanted to know how many people in each group had at least a 50% reduction in their monthly migraine headaches.Of the 580 people in the study, 287 were assigned to galcanezumab and 293 to rimegepant. In both groups, most were female and white. After 3 months of treatment, 62% of the people in the galcanezumab group and 61% of people in the rimegepant group had at least a 50% reduction in monthly migraine headaches. Both treatments were effective, but galcanezumab was not better than rimegepant. About 20% of the people in each treatment group had a side effect from the medication, and most were mild or moderate in severity.
© 2023. The Author(s).
Conflict of interest statement
Todd Schwedt has received compensation for
Figures

References
-
- Diener HC, Tfelt-Hansen P, Dahlof C, et al. Topiramate in migraine prophylaxis—results from a placebo-controlled trial with propranolol as an active control. J Neurol. 2004;251:943–50. - PubMed
-
- Ho TW, Ferrari MD, Dodick DW, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372(9656):2115–2123. doi: 10.1016/S0140-6736(08)61626-8. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

