Mechanisms of actin disassembly and turnover
- PMID: 37948068
- PMCID: PMC10638096
- DOI: 10.1083/jcb.202309021
Mechanisms of actin disassembly and turnover
Abstract
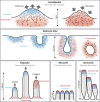
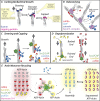
Cellular actin networks exhibit a wide range of sizes, shapes, and architectures tailored to their biological roles. Once assembled, these filamentous networks are either maintained in a state of polarized turnover or induced to undergo net disassembly. Further, the rates at which the networks are turned over and/or dismantled can vary greatly, from seconds to minutes to hours or even days. Here, we review the molecular machinery and mechanisms employed in cells to drive the disassembly and turnover of actin networks. In particular, we highlight recent discoveries showing that specific combinations of conserved actin disassembly-promoting proteins (cofilin, GMF, twinfilin, Srv2/CAP, coronin, AIP1, capping protein, and profilin) work in concert to debranch, sever, cap, and depolymerize actin filaments, and to recharge actin monomers for new rounds of assembly.
© 2023 Goode et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

