Identification and Optimization of Novel Inhibitors of the Polyketide Synthase 13 Thioesterase Domain with Antitubercular Activity
- PMID: 37948640
- PMCID: PMC10683028
- DOI: 10.1021/acs.jmedchem.3c01514
Identification and Optimization of Novel Inhibitors of the Polyketide Synthase 13 Thioesterase Domain with Antitubercular Activity
Abstract
There is an urgent need for new tuberculosis (TB) treatments, with novel modes of action, to reduce the incidence/mortality of TB and to combat resistance to current treatments. Through both chemical and genetic methodologies, polyketide synthase 13 (Pks13) has been validated as essential for mycobacterial survival and as an attractive target for Mycobacterium tuberculosis growth inhibitors. A benzofuran series of inhibitors that targeted the Pks13 thioesterase domain, failed to progress to preclinical development due to concerns over cardiotoxicity. Herein, we report the identification of a novel oxadiazole series of Pks13 inhibitors, derived from a high-throughput screening hit and structure-guided optimization. This new series binds in the Pks13 thioesterase domain, with a distinct binding mode compared to the benzofuran series. Through iterative rounds of design, assisted by structural information, lead compounds were identified with improved antitubercular potencies (MIC < 1 μM) and in vitro ADMET profiles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

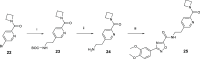
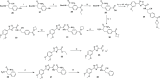
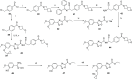

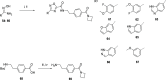

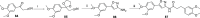
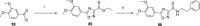

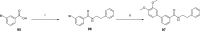
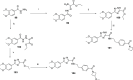

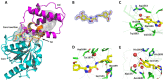
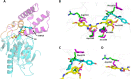
References
-
- WHO . Global tuberculosis report 2020; World Health Organization: Geneva, Switzerland, 2020.
-
- WHO . The end TB strategy, WHO/HTM/TB/2015.19; World Health Organization: Geneva, Switzerland, 2015.
-
- WHO . Global tuberculosis report 2022; World Health Organization: Geneva, Switzerland, 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

