Systematic review and meta-analysis of immune checkpoint inhibitors as single agent or in combination with chemotherapy in early-stage non-small cell lung cancer: Impact of clinicopathological factors and indirect comparison between treatment strategies
- PMID: 37948842
- PMCID: PMC12697757
- DOI: 10.1016/j.ejca.2023.113404
Systematic review and meta-analysis of immune checkpoint inhibitors as single agent or in combination with chemotherapy in early-stage non-small cell lung cancer: Impact of clinicopathological factors and indirect comparison between treatment strategies
Abstract
Background: In non-small cell lung cancer (NSCLC), the immune checkpoint inhibitors (ICI) revolution is rapidly moving from metastatic to early-stage, however, the impact of clinicopathological variables and optimal treatment sequencing remain unclear.
Methods: Randomized controlled trials (RCTs) in patients with early-stage NSCLC treated with ICI as single agent or in combination with platinum-based chemotherapy (PCT) were included. Primary outcomes were pathological complete response (pCR), event free survival (EFS) (neoadjuvant/perioperative), and disease-free survival (DFS) (adjuvant). Secondary outcomes were major pathological response (MPR), overall survival (OS), toxicity, surgical outcomes (neoadjuvant/perioperative); OS and toxicity (adjuvant). An additional secondary endpoint was to compare EFS and OS between neoadjuvant and perioperative strategies.
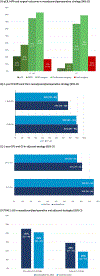
Results: 8 RCTs (2 neoadjuvant, 4 perioperative, 2 adjuvant) (4661 participants) were included. Neoadjuvant/perioperative ICI+PCT significantly improved pCR, EFS, OS, MPR and R0 resection compared to PCT. Adjuvant ICI significantly improved DFS compared to placebo. There was a significant subgroup interaction by PD-L1 status (χ2 = 10.72, P = 0.005), pCR (χ2 = 17.80, P < 0.0001), and stage (χ2 = 4.46, P = 0.003) for EFS. No difference according to PD-L1 status was found for pCR, with 14% of patients having PD-L1 negative tumors still experiencing a pCR. No interaction by PD-L1 status was found for DFS upon adjuvant ICI. Indirect comparison showed no difference in EFS and OS between neoadjuvant and perioperative ICI+PCT.
Conclusions: PD-L1 status, pCR and stage impact on survival upon neoadjuvant/perioperative ICI. The restriction of neoadjuvant/perioperative ICI to PD-L1 + patients could preclude pCR and long-term benefit in the PD-L1- subgroup. Neoadjuvant and perioperative could be equivalent strategies.
Keywords: Adjuvant; Early-stage; Immune checkpoint inhibitors; Meta-analysis; NSCLC; Neoadjuvant; pCR.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: T.C. reports (over the past 36 months) speaker fees/honoraria from the Society for Immunotherapy of Cancer (SITC), Mark Foundation for Cancer Research, Bristol Myers Squibb, Roche, Medscape, IDEOlogy Health, Physicians' Education Resource® LLC (PER®), OncLive, PeerView and Clinical Care Options (CCO); advisory role/consulting fees from MedImmune/AstraZeneca, Bristol Myers Squibb, Merck & Co., Genentech, Arrowhead Pharmaceuticals, Pfizer Inc. and Regeneron; institutional research funding from MedImmune/AstraZeneca and Bristol Myers Squibb; and travel, food and/or beverage expenses from Physicians' Education Resource® LLC (PER®), Dava Oncology, SITC, International Association for the Study of Lung Cancer, Parker Institute for Cancer Immunotherapy, IDEOlogy Health, OncLive, AstraZeneca and Bristol Myers Squibb.M.C.G. reports AstraZeneca, Abion, MSD International GmbH, Bayer, BMS, Boehringer Ingelheim Italia S.p.A, Celgene, Eli Lilly, Incyte, Novartis, Pfizer, Roche, Takeda, Seattle Genetics, Mirati, Daiichi Sankyo, Regeneron, Merck, Blueprint, Jansenn, Sanofi, AbbVie, BeiGenius, Oncohost, Medscape G.V. is consultant for Ab Medica, Roche, AstraZeneca, MSD, outside the submitted work R.F. reports advisory board for MSD and advisory board for BeiGene M.R. declares grants or contracts from AstraZeneca and participation on a Data Safety Monitoring Board or Advisory Board for Eli Lilly, PANAVANCE, Celgene, AstraZeneca, Viatris, Merck Sharp & Dohme, Servier, SOTIO, and Baxter. All remaining authors have declared no conflicts of interest.
Figures





References
-
- Besse B, Adam J, Cozic N, Chaput-Gras N, Planchard D, Mezquita L, et al. 1215O - SC Neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): results from the phase II PRINCEPS trial. Ann Oncol 2020;31:S794–5. 10.1016/j.annonc.2020.08.1417. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

