Systematic evidence mapping informs a class-based approach to assessing personal care products and pubertal timing
- PMID: 37948866
- PMCID: PMC12368981
- DOI: 10.1016/j.envint.2023.108307
Systematic evidence mapping informs a class-based approach to assessing personal care products and pubertal timing
Abstract
Background: Personal care products (PCPs) contain many different compounds and are a source of exposure to endocrine disrupting chemicals (EDCs), including phthalates and phenols. Early-life exposure to EDCs commonly found in PCPs has been linked to earlier onset of puberty.
Objective: To characterize the human and animal evidence on the association between puberty-related outcomes and exposure to PCPs and their chemical constituents and, if there is sufficient evidence, identify groups of chemicals and outcomes to support a systematic review for a class-based hazard or risk assessment.
Methods: We followed the OHAT systematic review framework to characterize the human and animal evidence on the association between puberty-related health outcomes and exposure to PCPs and their chemical constituents.
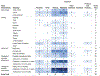
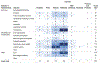
Results: Ninety-eight human and 299 animal studies that evaluated a total of 96 different chemicals were identified and mapped by key concepts including chemical class, data stream, and puberty-related health outcome. Among these studies, phthalates and phenols were the most well-studied chemical classes. Most of the phthalate and phenol studies examined secondary sex characteristics and changes in estradiol and testosterone levels. Studies evaluating PCP use and other chemical classes (e.g., parabens) had less data.
Conclusions: This systematic evidence map identified and mapped the published research evaluating the association between exposure to PCPs and their chemical constituents and puberty-related health outcomes. The resulting interactive visualization allows researchers to make evidence-based decisions on the available research by enabling them to search, sort, and filter the literature base of puberty-related studies by key concepts. This map can be used by researchers and regulators to prioritize and target future research and funding to reduce uncertainties and address data gaps. It also provides information to inform a class-based hazard or risk assessment on the association between phthalate and phenol exposures and puberty-related health outcomes.
Keywords: Chemical class; Personal care products; Phenols; Phthalates; Puberty; Systematic evidence map.
Copyright © 2023. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



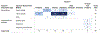
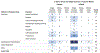

References
-
- 117th Congress. No PFAS in Cosmetics Act. 2021
-
- Ashrap P; Sánchez BN; Téllez-Rojo MM; Basu N; Tamayo-Ortiz M; Peterson KE; Meeker JD; Watkins DJ In utero and peripubertal metals exposure in relation to reproductive hormones and sexual maturation and progression among girls in Mexico City. Environ Res. 2019;177:108630. 10.1016/j.envres.2019.108630 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

