Proteostasis governs differential temperature sensitivity across embryonic cell types
- PMID: 37949057
- PMCID: PMC11178971
- DOI: 10.1016/j.cell.2023.10.013
Proteostasis governs differential temperature sensitivity across embryonic cell types
Abstract
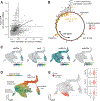
Embryonic development is remarkably robust, but temperature stress can degrade its ability to generate animals with invariant anatomy. Phenotypes associated with environmental stress suggest that some cell types are more sensitive to stress than others, but the basis of this sensitivity is unknown. Here, we characterize hundreds of individual zebrafish embryos under temperature stress using whole-animal single-cell RNA sequencing (RNA-seq) to identify cell types and molecular programs driving phenotypic variability. We find that temperature perturbs the normal proportions and gene expression programs of numerous cell types and also introduces asynchrony in developmental timing. The notochord is particularly sensitive to temperature, which we map to a specialized cell type: sheath cells. These cells accumulate misfolded protein at elevated temperature, leading to a cascading structural failure of the notochord and anatomic defects. Our study demonstrates that whole-animal single-cell RNA-seq can identify mechanisms for developmental robustness and pinpoint cell types that constitute key failure points.
Keywords: developmental robustness; single-cell RNA-seq; variability; zebrafish.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.T. is a scientific advisory board (SAB) member, consultant, and/or co-founder of Algen Biotechnologies, Altius Therapeutics, and Scale Biosciences. J.S. is an SAB member, consultant, and/or co-founder of Cajal Neuroscience, Guardant Health, Maze Therapeutics, Camp4 Therapeutics, Phase Genomics, Adaptive Biotechnologies, and Scale Biosciences.
Figures
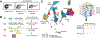



References
-
- Schirone RC, and Gross L (1968). Effect of temperature on early embryological development of the zebra fish,Brachydanio rerio. J. Exp. Zool. 169, 43–52.
-
- Sharpe PJ, and DeMichele DW (1977). Reaction kinetics of poikilotherm development. J. Theor. Biol. 64, 649–670. - PubMed
-
- Gillooly JF, Charnov EL, West GB, Savage VM, and Brown JH (2002). Effects of size and temperature on developmental time. Nature 417, 70–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

