Cardioprotective O-GlcNAc signaling is elevated in murine female hearts via enhanced O-GlcNAc transferase activity
- PMID: 37949223
- PMCID: PMC10711226
- DOI: 10.1016/j.jbc.2023.105447
Cardioprotective O-GlcNAc signaling is elevated in murine female hearts via enhanced O-GlcNAc transferase activity
Abstract
The post-translational modification of intracellular proteins by O-linked β-GlcNAc (O-GlcNAc) has emerged as a critical regulator of cardiac function. Enhanced O-GlcNAcylation activates cytoprotective pathways in cardiac models of ischemia-reperfusion (I/R) injury; however, the mechanisms underpinning O-GlcNAc cycling in response to I/R injury have not been comprehensively assessed. The cycling of O-GlcNAc is regulated by the collective efforts of two enzymes: O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), which catalyze the addition and hydrolysis of O-GlcNAc, respectively. It has previously been shown that baseline heart physiology and pathophysiology are impacted by sex. Here, we hypothesized that sex differences in molecular signaling may target protein O-GlcNAcylation both basally and in ischemic hearts. To address this question, we subjected male and female WT murine hearts to ex vivo ischemia or I/R injury. We assessed hearts for protein O-GlcNAcylation, abundance of OGT, OGA, and glutamine:fructose-6-phosphate aminotransferase (GFAT2), activity of OGT and OGA, and UDP-GlcNAc levels. Our data demonstrate elevated O-GlcNAcylation in female hearts both basally and during ischemia. We show that OGT activity was enhanced in female hearts in all treatments, suggesting a mechanism for these observations. Furthermore, we found that ischemia led to reduced O-GlcNAcylation and OGT-specific activity. Our findings provide a foundation for understanding molecular mechanisms that regulate O-GlcNAcylation in the heart and highlight the importance of sex as a significant factor when assessing key regulatory events that control O-GlcNAc cycling. These data suggest the intriguing possibility that elevated O-GlcNAcylation in females contributes to reduced ischemic susceptibility.
Keywords: O-GlcNAc; O-GlcNAc transferase; O-GlcNAcase; cardiac; glycosylation; hexosamine biosynthetic pathway; sex differences.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


 ), and the duration of contracture (
), and the duration of contracture ( ) between males and females. Statistical test: two-way ANOVA. ∗∗∗p < 0.001. Data are represented as mean ± SD.
) between males and females. Statistical test: two-way ANOVA. ∗∗∗p < 0.001. Data are represented as mean ± SD.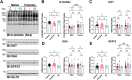
 ]; I/R 20 min ischemia/120 min reperfusion [
]; I/R 20 min ischemia/120 min reperfusion [ ]). Hearts were extracted in 1% N-P40 buffer, and proteins were separated by SDS-PAGE (15 or 22.5 μg), electroblotted to nitrocellulose, and the following were detected: OGT, OGA, GFAT2, heat shock cognate 70 (Hsc70), O-GlcNAc (RL2), and O-GlcNAc specificity control (RL2 with 500 mM GlcNAc, not shown). Hsc70 was used as a representative immunoblot for protein load. Protein load was assessed by total protein membrane stain (SYPRO Ruby or Direct Blue-71, Fig. S2). The following were quantified and normalized to total protein. (B) O-GlcNAc (RL2); (C) OGT; (D) OGA; and (E) GFAT2. B–E, pooled male and female data are reported on the left. Statistical test: one-way ANOVA. ∗p < 0.05. Data parsed by sex are reported on the right. Statistical test: two-way ANOVA. ∗∗p < 0.01; ∗∗∗∗p < 0.0001. Data are represented as mean ± SD. GFAT2, glutamine-fructose 6 phosphate amidotransferase 2; IR, I/R, ischemia–reperfusion; OGA, O-GlcNAcase; O-GlcNAc, O-linked β-GlcNAc; OGT, O-GlcNAc transferase.
]). Hearts were extracted in 1% N-P40 buffer, and proteins were separated by SDS-PAGE (15 or 22.5 μg), electroblotted to nitrocellulose, and the following were detected: OGT, OGA, GFAT2, heat shock cognate 70 (Hsc70), O-GlcNAc (RL2), and O-GlcNAc specificity control (RL2 with 500 mM GlcNAc, not shown). Hsc70 was used as a representative immunoblot for protein load. Protein load was assessed by total protein membrane stain (SYPRO Ruby or Direct Blue-71, Fig. S2). The following were quantified and normalized to total protein. (B) O-GlcNAc (RL2); (C) OGT; (D) OGA; and (E) GFAT2. B–E, pooled male and female data are reported on the left. Statistical test: one-way ANOVA. ∗p < 0.05. Data parsed by sex are reported on the right. Statistical test: two-way ANOVA. ∗∗p < 0.01; ∗∗∗∗p < 0.0001. Data are represented as mean ± SD. GFAT2, glutamine-fructose 6 phosphate amidotransferase 2; IR, I/R, ischemia–reperfusion; OGA, O-GlcNAcase; O-GlcNAc, O-linked β-GlcNAc; OGT, O-GlcNAc transferase.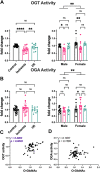
 ]; I/R 20 min ischemia/120 min reperfusion [
]; I/R 20 min ischemia/120 min reperfusion [ ]). Hearts were extracted in 1% NP-40, and equal protein was desalted using a Zebaspin column. A, OGT activity was assessed by measuring transfer of 3H-GlcNAc to the casein kinase II (CK2) acceptor peptide. B, OGA activity was detected using the pseudosubstrate 4-methylumbelliferyl-β-GlcNAc. A and B, pooled male and female data are reported on the left. Statistical test: one-way ANOVA. ∗∗p < 0.01; ∗∗∗∗p < 0.0001. Data parsed by sex are reported on the right. Statistical test: two-way ANOVA. ∗p < 0.05; ∗∗p < 0.01. Data are represented as mean ± SD. C, correlation of OGT activity and O-GlcNAc (RL2). D, correlation of OGA activity and O-GlcNAc (RL2). C and D, r = Pearson correlation coefficient. I/R, ischemia–reperfusion; OGA, O-GlcNAcase; OGT, O-GlcNAc transferase.
]). Hearts were extracted in 1% NP-40, and equal protein was desalted using a Zebaspin column. A, OGT activity was assessed by measuring transfer of 3H-GlcNAc to the casein kinase II (CK2) acceptor peptide. B, OGA activity was detected using the pseudosubstrate 4-methylumbelliferyl-β-GlcNAc. A and B, pooled male and female data are reported on the left. Statistical test: one-way ANOVA. ∗∗p < 0.01; ∗∗∗∗p < 0.0001. Data parsed by sex are reported on the right. Statistical test: two-way ANOVA. ∗p < 0.05; ∗∗p < 0.01. Data are represented as mean ± SD. C, correlation of OGT activity and O-GlcNAc (RL2). D, correlation of OGA activity and O-GlcNAc (RL2). C and D, r = Pearson correlation coefficient. I/R, ischemia–reperfusion; OGA, O-GlcNAcase; OGT, O-GlcNAc transferase.
 ]; I/R 20 min ischemia/120 min reperfusion [
]; I/R 20 min ischemia/120 min reperfusion [ ]). Heart nucleotides and nucleotide sugars were extracted in methanol:chloroform, desalted using solid-phase extraction, and analyzed using high-performance anion-exchange chromatography. Nucleotide/nucleotide sugar levels were normalized to total protein: (A) UDP-GlcNAc; (B) ATP; (C) ADP; (D) AMP. A–D, pooled male and female data are reported on the left. Statistical test: one-way ANOVA. ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗∗p < 0.0001. Data parsed by sex are reported on the right. Statistical test: two-way ANOVA. ∗p < 0.05; ∗∗p < 0.01. Data are represented as mean ± SD. E, representative separation of nucleotide sugars. Peaks are numbered as follows: (1) CMP, CMP-neuraminic acid; (2) AMP; (3) UMP; (4) UDP-GlcNAc; (5) UDP-GalNAc; (6) UDP-glucose, UDP-galactose, UDP-xylose, CDP, GMP; (7) ADP; (8) UDP, GDP-mannose, GDP-fucose, CTP, UTP; (9) ATP, GDP; and (10) UDP-glucuronic acid. The sodium acetate gradient used to achieve nucleotide separation is depicted on the right Y-axis. F, correlation of UDP-GlcNAc and O-GlcNAc (RL2). G, correlation of AMP:ATP ratio and OGT activity. F and G, r = Pearson's correlation coefficient. I/R, ischemia–reperfusion.
]). Heart nucleotides and nucleotide sugars were extracted in methanol:chloroform, desalted using solid-phase extraction, and analyzed using high-performance anion-exchange chromatography. Nucleotide/nucleotide sugar levels were normalized to total protein: (A) UDP-GlcNAc; (B) ATP; (C) ADP; (D) AMP. A–D, pooled male and female data are reported on the left. Statistical test: one-way ANOVA. ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗∗p < 0.0001. Data parsed by sex are reported on the right. Statistical test: two-way ANOVA. ∗p < 0.05; ∗∗p < 0.01. Data are represented as mean ± SD. E, representative separation of nucleotide sugars. Peaks are numbered as follows: (1) CMP, CMP-neuraminic acid; (2) AMP; (3) UMP; (4) UDP-GlcNAc; (5) UDP-GalNAc; (6) UDP-glucose, UDP-galactose, UDP-xylose, CDP, GMP; (7) ADP; (8) UDP, GDP-mannose, GDP-fucose, CTP, UTP; (9) ATP, GDP; and (10) UDP-glucuronic acid. The sodium acetate gradient used to achieve nucleotide separation is depicted on the right Y-axis. F, correlation of UDP-GlcNAc and O-GlcNAc (RL2). G, correlation of AMP:ATP ratio and OGT activity. F and G, r = Pearson's correlation coefficient. I/R, ischemia–reperfusion.References
-
- Tsao C.W., Aday A.W., Almarzooq Z.I., Anderson C.A.M., Arora P., Avery C.L., et al. Heart disease and stroke statistics—2023 update: a report from the American heart association. Circulation. 2023;147:e93–e621. - PubMed
-
- Peters H.W., Westendorp I.C.D., Hak A.E., Grobbee D.E., Stehouwer C.D.A., Hofman A., et al. Menopausal status and risk factors for cardiovascular disease. J. Intern. Med. 1999;246:521–528. - PubMed
-
- Cagnacci A., Cannoletta M., Palma F., Zanin R., Xholli A., Volpe A. Menopausal symptoms and risk factors for cardiovascular disease in postmenopause. Climacteric. 2012;15:157–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

