Weathering the Storm: Syringe Services Program Laws and Human Immunodeficiency Virus During the COVID-19 Pandemic
- PMID: 37949442
- PMCID: PMC11299426
- DOI: 10.1097/QAI.0000000000003293
Weathering the Storm: Syringe Services Program Laws and Human Immunodeficiency Virus During the COVID-19 Pandemic
Abstract
Background: Syringe services programs (SSPs) are community-based prevention programs that provide a range of harm reduction services to persons who inject drugs. Despite their benefits, SSP laws vary across the United States. Little is known regarding how legislation surrounding SSPs may have influenced HIV transmission over the COVID-19 pandemic, a period in which drug use increased. This study examined associations between state SSP laws and HIV transmission among the Medicaid population before and after the COVID-19 pandemic.
Methods: State-by-month counts of new HIV diagnoses among the Medicaid population were produced using administrative claims data from the Transformed Medicaid Statistical Information System from 2019 to 2020. Data on SSP laws were collected from the Prescription Drug Abuse Policy System. Associations between state SSP laws and HIV transmission before and after the start of the COVID-19 pandemic were evaluated using an event study design, controlling for the implementation of COVID-19 nonpharmaceutical interventions and state and time fixed effects.
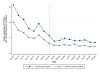
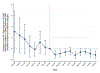
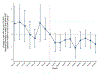
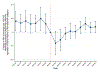
Results: State laws allowing the operation of SSPs were associated with 0.54 (P = 0.044) to 1.18 (P = 0.001) fewer new monthly HIV diagnoses per 100,000 Medicaid enrollees relative to states without such laws in place during the 9 months after the start of the COVID-19 pandemic. The largest effects manifested for population subgroups disproportionately affected by HIV, such as male and non-Hispanic Black Medicaid enrollees.
Conclusion: Less restrictive laws on SSPs may have helped mitigate HIV transmission among the Medicaid population throughout the COVID-19 pandemic. Policymakers can consider implementing less restrictive SSP laws to mitigate HIV transmission resulting from future increases in injection drug use.
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors have no funding or conflicts of interest to disclose.
Figures
References
-
- Glenshaw MT, Gaist P, Wilson A, Cregg RC, Holtz TH, & Goodenow MM. Role of NIH in the Ending the HIV Epidemic in the US Initiative: Research Improving Practice. JAIDS Journal of Acquired Immune Deficiency Syndromes, 2022;90(1):S9–S16. - PubMed
-
- Centers for Disease Control and Prevention. HIV: Basic Statistics [internet]. Updated 6-21-2022. Accessed on 8-1-2022. Accessed from https://www.cdc.gov/hiv/basics/statistics.html.
-
- Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2020. HIV Surveillance Report, 2020; vol. 33. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2022. Accessed [8-1-2022].
-
- Hulsey J, Mellis A, Kelly B. COVID-19 pandemic impact on patients, families and individuals in recovery from substance use disorders. Addiction Policy Forum. https://www.researchgate.net/profile/Jessica-Hulsey/publication/36137138.... Published 6-9-2020. Accessed 8-16-2022.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

