Multiple Concurrent Predictions Inform Prediction Error in the Human Auditory Pathway
- PMID: 37949655
- PMCID: PMC10851690
- DOI: 10.1523/JNEUROSCI.2219-22.2023
Multiple Concurrent Predictions Inform Prediction Error in the Human Auditory Pathway
Abstract
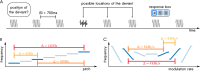
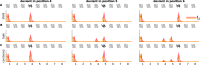
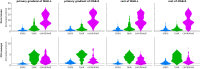
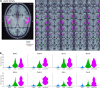
The key assumption of the predictive coding framework is that internal representations are used to generate predictions on how the sensory input will look like in the immediate future. These predictions are tested against the actual input by the so-called prediction error units, which encode the residuals of the predictions. What happens to prediction errors, however, if predictions drawn by different stages of the sensory hierarchy contradict each other? To answer this question, we conducted two fMRI experiments while female and male human participants listened to sequences of sounds: pure tones in the first experiment and frequency-modulated sweeps in the second experiment. In both experiments, we used repetition to induce predictions based on stimulus statistics (stats-informed predictions) and abstract rules disclosed in the task instructions to induce an orthogonal set of (task-informed) predictions. We tested three alternative scenarios: neural responses in the auditory sensory pathway encode prediction error with respect to (1) the stats-informed predictions, (2) the task-informed predictions, or (3) a combination of both. Results showed that neural populations in all recorded regions (bilateral inferior colliculus, medial geniculate body, and primary and secondary auditory cortices) encode prediction error with respect to a combination of the two orthogonal sets of predictions. The findings suggest that predictive coding exploits the non-linear architecture of the auditory pathway for the transmission of predictions. Such non-linear transmission of predictions might be crucial for the predictive coding of complex auditory signals like speech.Significance Statement Sensory systems exploit our subjective expectations to make sense of an overwhelming influx of sensory signals. It is still unclear how expectations at each stage of the processing pipeline are used to predict the representations at the other stages. The current view is that this transmission is hierarchical and linear. Here we measured fMRI responses in auditory cortex, sensory thalamus, and midbrain while we induced two sets of mutually inconsistent expectations on the sensory input, each putatively encoded at a different stage. We show that responses at all stages are concurrently shaped by both sets of expectations. The results challenge the hypothesis that expectations are transmitted linearly and provide for a normative explanation of the non-linear physiology of the corticofugal sensory system.
Keywords: auditory midbrain; auditory pathway; cortico-thalamic interactions; predictive coding; sensory processing; sensory thalamus.
Copyright © 2023 the authors.
Figures





Similar articles
-
Adjudicating Between Local and Global Architectures of Predictive Processing in the Subcortical Auditory Pathway.Front Neural Circuits. 2021 Mar 12;15:644743. doi: 10.3389/fncir.2021.644743. eCollection 2021. Front Neural Circuits. 2021. PMID: 33776657 Free PMC article. Review.
-
Corticofugal regulation of predictive coding.Elife. 2022 Mar 15;11:e73289. doi: 10.7554/eLife.73289. Elife. 2022. PMID: 35290181 Free PMC article.
-
Stimulus-Specific Prediction Error Neurons in Mouse Auditory Cortex.J Neurosci. 2023 Oct 25;43(43):7119-7129. doi: 10.1523/JNEUROSCI.0512-23.2023. Epub 2023 Sep 12. J Neurosci. 2023. PMID: 37699716 Free PMC article.
-
Mapping Frequency-Specific Tone Predictions in the Human Auditory Cortex at High Spatial Resolution.J Neurosci. 2018 May 23;38(21):4934-4942. doi: 10.1523/JNEUROSCI.2205-17.2018. Epub 2018 Apr 30. J Neurosci. 2018. PMID: 29712781 Free PMC article.
-
Top-Down Inference in the Auditory System: Potential Roles for Corticofugal Projections.Front Neural Circuits. 2021 Jan 22;14:615259. doi: 10.3389/fncir.2020.615259. eCollection 2020. Front Neural Circuits. 2021. PMID: 33551756 Free PMC article. Review.
Cited by
-
Two Prediction Error Systems in the Nonlemniscal Inferior Colliculus: "Spectral" and "Nonspectral".J Neurosci. 2024 Jun 5;44(23):e1420232024. doi: 10.1523/JNEUROSCI.1420-23.2024. J Neurosci. 2024. PMID: 38627089 Free PMC article.
-
Cortical-subcortical interactions underlie processing of auditory predictions measured with 7T fMRI.Cereb Cortex. 2024 Aug 1;34(8):bhae316. doi: 10.1093/cercor/bhae316. Cereb Cortex. 2024. PMID: 39087881 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
