Biodistribution and function of coupled polymer-DNA origami nanostructures
- PMID: 37949918
- PMCID: PMC10638432
- DOI: 10.1038/s41598-023-46351-1
Biodistribution and function of coupled polymer-DNA origami nanostructures
Abstract
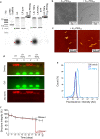
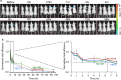
Spatial control over the distribution of therapeutics is a highly desired feature, which could limit the side effects of many drugs. Here we describe a nanoscale agent, fabricated from a coupled polymer-DNA origami hybrid that exhibits stability in serum and slow diffusion through tissues, in a manner correlating with shape and aspect ratio. Coupling to fragments of polyethylene glycol (PEG) through polyamine electrostatic interactions resulted in marked stability of the agents in-vivo, with > 90% of the agents maintaining structural integrity 5 days following subcutaneous injection. An agent functionalized with aptamers specific for human tumor necrosis factor TNF-alpha, significantly abrogated the inflammatory response in a delayed-type hypersensitivity model in humanized TNF-alpha mice. These findings highlight polymer-DNA hybrid nanostructures as a programmable and pharmacologically viable update to mainstream technologies such as monoclonal antibodies, capable of exerting an additional layer of control across the spatial dimension of drug activity.
© 2023. The Author(s).
Conflict of interest statement
NJ, AS, EG, SB, AAH, and IB are currently (or were in the past) employees and/or shareholders in Augmanity, a research company based in Rehovot, Israel, that develops DNA-related technologies, and are listed as inventors on patents related to technologies reported in this paper. BM declares no financial interests. All authors declare no non-financial interests.
Figures



References
-
- Gilead Announces Partial Clinical Hold for Studies Evaluating Magrolimab in Combination With Azacitidine. https://www.gilead.com/news-and-press/press-room/press-releases/2022/1/g....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

