Exploring the known chemical space of the plant kingdom: insights into taxonomic patterns, knowledge gaps, and bioactive regions
- PMID: 37950325
- PMCID: PMC10636812
- DOI: 10.1186/s13321-023-00778-w
Exploring the known chemical space of the plant kingdom: insights into taxonomic patterns, knowledge gaps, and bioactive regions
Abstract
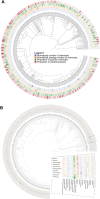
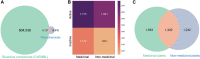
Plants are one of the primary sources of natural products for drug development. However, despite centuries of research, only a limited region of the phytochemical space has been studied. To understand the scope of what is explored versus unexplored in the phytochemical space, we begin by reconstructing the known chemical space of the plant kingdom, mapping the distribution of secondary metabolites, chemical classes, and plants traditionally used for medicinal purposes (i.e., medicinal plants) across various levels of the taxonomy. We identify hotspot taxonomic clades occupied by a large proportion of medicinal plants and characterized secondary metabolites, as well as clades requiring further characterization with regard to their chemical composition. In a complementary analysis, we build a chemotaxonomy which has a high level of concordance with the taxonomy at the genus level, highlighting the close relationship between chemical profiles and evolutionary relationships within the plant kingdom. Next, we delve into regions of the phytochemical space with known bioactivity that have been used in modern drug discovery. While we find that the vast majority of approved drugs from phytochemicals are derived from known medicinal plants, we also show that medicinal and non-medicinal plants do not occupy distinct regions of the known phytochemical landscape and their phytochemicals exhibit properties similar to bioactive compounds. Moreover, we also reveal that only a few thousand phytochemicals have been screened for bioactivity and that there are hundreds of known bioactive compounds present in both medicinal and non-medicinal plants, suggesting that non-medicinal plants also have potential therapeutic applications. Overall, these results support the hypothesis that there are many plants with medicinal properties awaiting discovery.
Keywords: Chemotaxonomy; Drug discovery; Natural products; Phytochemistry.
© 2023. The Author(s).
Conflict of interest statement
All authors were employees of Enveda Biosciences Inc. during the course of this work and have real or potential ownership interest in the company.
Figures


References
-
- Howes MJR, Quave CL, Collemare J, Tatsis EC, Twilley D, Lulekal E, et al. Molecules from nature: reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet. 2020;2(5):463–481. doi: 10.1002/ppp3.10138. - DOI
-
- Ncube B, Finnie JF, Van Staden J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S Afr J Bot. 2012;82:11–20. doi: 10.1016/j.sajb.2012.05.009. - DOI
LinkOut - more resources
Full Text Sources

