Skeletal muscle regeneration failure in ischemic-damaged limbs is associated with pro-inflammatory macrophages and premature differentiation of satellite cells
- PMID: 37950327
- PMCID: PMC10636829
- DOI: 10.1186/s13073-023-01250-y
Skeletal muscle regeneration failure in ischemic-damaged limbs is associated with pro-inflammatory macrophages and premature differentiation of satellite cells
Abstract
Background: Chronic limb-threatening ischemia (CLTI), a severe manifestation of peripheral arterial disease (PAD), is associated with a 1-year limb amputation rate of approximately 15-20% and substantial mortality. A key feature of CLTI is the compromised regenerative ability of skeletal muscle; however, the mechanisms responsible for this impairment are not yet fully understood. In this study, we aim to delineate pathological changes at both the cellular and transcriptomic levels, as well as in cell-cell signaling pathways, associated with compromised muscle regeneration in limb ischemia in both human tissue samples and murine models of CLTI.
Methods: We performed single-cell transcriptome analysis of ischemic and non-ischemic muscle from the same CLTI patients and from a murine model of CLTI. In both datasets, we analyzed gene expression changes in macrophage and muscle satellite cell (MuSC) populations as well as differential cell-cell signaling interactions and differentiation trajectories.
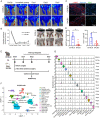
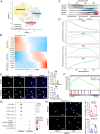
Results: Single-cell transcriptomic profiling and immunofluorescence analysis of CLTI patient skeletal muscle demonstrated that ischemic-damaged tissue displays a pro-inflammatory macrophage signature. Comparable results were observed in a murine CLTI model. Moreover, integrated analyses of both human and murine datasets revealed premature differentiation of MuSCs to be a key feature of failed muscle regeneration in the ischemic limb. Furthermore, in silico inferences of intercellular communication and in vitro assays highlight the importance of macrophage-MuSC signaling in ischemia induced muscle injuries.
Conclusions: Collectively, our research provides the first single-cell transcriptome atlases of skeletal muscle from CLTI patients and a murine CLTI model, emphasizing the crucial role of macrophages and inflammation in regulating muscle regeneration in CLTI through interactions with MuSCs.
Keywords: Chronic limb-threatening ischemia; Macrophage polarization; Murine hindlimb ischemia; Muscle satellite cells; Peripheral arterial disease; Single-cell transcriptome analysis; Skeletal muscle regeneration.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Update of
-
Pro-inflammatory macrophages impair skeletal muscle regeneration in ischemic-damaged limbs by inducing precocious differentiation of satellite cells.bioRxiv [Preprint]. 2023 Apr 3:2023.04.01.535211. doi: 10.1101/2023.04.01.535211. bioRxiv. 2023. Update in: Genome Med. 2023 Nov 10;15(1):95. doi: 10.1186/s13073-023-01250-y. PMID: 37066299 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

