Contemporary Management of Staphylococcus aureus Bacteremia-Controversies in Clinical Practice
- PMID: 37950887
- PMCID: PMC11959183
- DOI: 10.1093/cid/ciad500
Contemporary Management of Staphylococcus aureus Bacteremia-Controversies in Clinical Practice
Abstract
Staphylococcus aureus bacteremia (SAB) carries a high risk for excess morbidity and mortality. Despite its prevalence, significant practice variation continues to permeate clinical management of this syndrome. Since the publication of the 2011 Infectious Diseases Society of America (IDSA) guidelines on management of methicillin-resistant Staphylococcus aureus infections, the field of SAB has evolved with the emergence of newer diagnostic strategies and therapeutic options. In this review, we seek to provide a comprehensive overview of the evaluation and management of SAB, with special focus on areas where the highest level of evidence is lacking to inform best practices.
Keywords: Staphylococcus aureus bacteremia.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. D. J. M. reports payment from the Antimicrobial Resistance Leadership Group for participation in a systematic review and travel support from the University of California, San Francisco (UCSF), ID Division to attend IDWeek. H. F. C. reports an institutional grant from the National Institutes of Health/National Institute on Allergy and Infectious Diseases (NIH/NIAID) for the Antibacterial Resistance Leadership Group (UM1AI104681); royalties earned as editor for The Sanford Guide to Antimicrobial Therapy; payment for expert testimony in a patent dispute for Nexus Pharmaceuticals and a product liability case for Eli Lilly; support from Merck for participation in a Data and Safety Monitoring or Advisory Board; and stock in Moderna and Merck (spouse's IRA). S. B. D. reports funding for clinical research studies from Gilead, Pfizer, F2G, Regeneron, Chan Zuckerberg Biohub, and NIAID; personal consulting fees from Genentech and Janssen/J+J; travel support from IDSA to speak at IDWeek; a patent for Mif agonists and antagonist and therapeutic uses (US20100143379A1); roles on the IDSA Antibacterial Resistance Committee, CADPH HAI Advisory Committee, Antibacterial Resistance Leadership Group Innovations Group, Laboratory Center, Mentorship Committee, Gram Positive Committee, and Immunosuppressed Host Group; and compensation for clinical events committee participation from Shinogi, Basilea, and Duke Clinical Research Institute. A. A. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
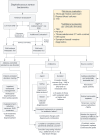
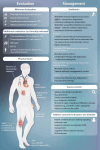
References
-
- King HA, Doernberg SB, Miller J, et al. Patients' experiences with Staphylococcus aureus and gram-negative bacterial bloodstream infections: a qualitative descriptive study and concept elicitation phase to inform measurement of patient-reported quality of life. Clin Infect Dis 2021; 73:237–47. - PMC - PubMed
-
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. - PubMed

