The translational oscillation in oocyte and early embryo development
- PMID: 37950888
- PMCID: PMC10711566
- DOI: 10.1093/nar/gkad996
The translational oscillation in oocyte and early embryo development
Abstract
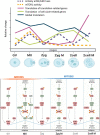
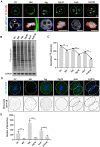
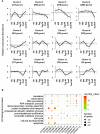
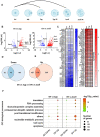
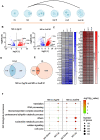
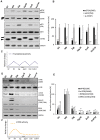
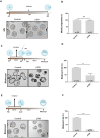
Translation is critical for development as transcription in the oocyte and early embryo is silenced. To illustrate the translational changes during meiosis and consecutive two mitoses of the oocyte and early embryo, we performed a genome-wide translatome analysis. Acquired data showed significant and uniform activation of key translational initiation and elongation axes specific to M-phases. Although global protein synthesis decreases in M-phases, translation initiation and elongation activity increases in a uniformly fluctuating manner, leading to qualitative changes in translation regulation via the mTOR1/4F/eEF2 axis. Overall, we have uncovered a highly dynamic and oscillatory pattern of translational reprogramming that contributes to the translational regulation of specific mRNAs with different modes of polysomal occupancy/translation that are important for oocyte and embryo developmental competence. Our results provide new insights into the regulation of gene expression during oocyte meiosis as well as the first two embryonic mitoses and show how temporal translation can be optimized. This study is the first step towards a comprehensive analysis of the molecular mechanisms that not only control translation during early development, but also regulate translation-related networks employed in the oocyte-to-embryo transition and embryonic genome activation.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

