Aspirin and Hemocompatibility Events With a Left Ventricular Assist Device in Advanced Heart Failure: The ARIES-HM3 Randomized Clinical Trial
- PMID: 37950897
- PMCID: PMC10640705
- DOI: 10.1001/jama.2023.23204
Aspirin and Hemocompatibility Events With a Left Ventricular Assist Device in Advanced Heart Failure: The ARIES-HM3 Randomized Clinical Trial
Abstract
Importance: Left ventricular assist devices (LVADs) enhance quality and duration of life in advanced heart failure. The burden of nonsurgical bleeding events is a leading morbidity. Aspirin as an antiplatelet agent is mandated along with vitamin K antagonists (VKAs) with continuous-flow LVADs without conclusive evidence of efficacy and safety.
Objective: To determine whether excluding aspirin as part of the antithrombotic regimen with a fully magnetically levitated LVAD is safe and decreases bleeding.
Design, setting, and participants: This international, randomized, double-blind, placebo-controlled study of aspirin (100 mg/d) vs placebo with VKA therapy in patients with advanced heart failure with an LVAD was conducted across 51 centers with expertise in treating patients with advanced heart failure across 9 countries. The randomized population included 628 patients with advanced heart failure implanted with a fully magnetically levitated LVAD (314 in the placebo group and 314 in the aspirin group), of whom 296 patients in the placebo group and 293 in the aspirin group were in the primary analysis population, which informed the primary end point analysis. The study enrolled patients from July 2020 to September 2022; median follow-up was 14 months.
Intervention: Patients were randomized in a 1:1 ratio to receive aspirin (100 mg/d) or placebo in addition to an antithrombotic regimen.
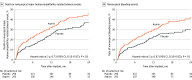
Main outcomes and measures: The composite primary end point, assessed for noninferiority (-10% margin) of placebo, was survival free of a major nonsurgical (>14 days after implant) hemocompatibility-related adverse events (including stroke, pump thrombosis, major bleeding, or arterial peripheral thromboembolism) at 12 months. The principal secondary end point was nonsurgical bleeding events.
Results: Of the 589 analyzed patients, 77% were men; one-third were Black and 61% were White. More patients were alive and free of hemocompatibility events at 12 months in the placebo group (74%) vs those taking aspirin (68%). Noninferiority of placebo was demonstrated (absolute between-group difference, 6.0% improvement in event-free survival with placebo [lower 1-sided 97.5% CI, -1.6%]; P < .001). Aspirin avoidance was associated with reduced nonsurgical bleeding events (relative risk, 0.66 [95% confidence limit, 0.51-0.85]; P = .002) with no increase in stroke or other thromboembolic events, a finding consistent among diverse subgroups of patient characteristics.
Conclusions and relevance: In patients with advanced heart failure treated with a fully magnetically levitated LVAD, avoidance of aspirin as part of an antithrombotic regimen, which includes VKA, is not inferior to a regimen containing aspirin, does not increase thromboembolism risk, and is associated with a reduction in bleeding events.
Trial registration: ClinicalTrials.gov Identifier: NCT04069156.
Conflict of interest statement
Figures


Comment in
-
Aspirin exclusion in patients with an LVAD.Nat Rev Cardiol. 2024 Feb;21(2):72. doi: 10.1038/s41569-023-00965-0. Nat Rev Cardiol. 2024. PMID: 38012304 No abstract available.
Comment on
-
Addition by Subtraction in Mechanical Cardiac Support.JAMA. 2023 Dec 12;330(22):2165-2166. doi: 10.1001/jama.2023.22490. JAMA. 2023. PMID: 37950896 No abstract available.
References
-
- Uriel N, Colombo PC, Cleveland JC, et al. . Hemocompatibility-related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation. 2017;135(21):2003-2012. doi:10.1161/CIRCULATIONAHA.117.028303 - DOI - PubMed

