Multidisciplinary clinical guidelines in proactive monitoring, early diagnosis, and effective management of trastuzumab deruxtecan (T-DXd)-induced interstitial lung disease (ILD) in breast cancer patients
- PMID: 37951130
- PMCID: PMC10679891
- DOI: 10.1016/j.esmoop.2023.102043
Multidisciplinary clinical guidelines in proactive monitoring, early diagnosis, and effective management of trastuzumab deruxtecan (T-DXd)-induced interstitial lung disease (ILD) in breast cancer patients
Abstract
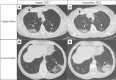
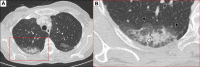
Trastuzumab deruxtecan (T-DXd), a human epidermal growth factor receptor 2 (HER2)-directed antibody-drug conjugate (ADC), has altered the treatment landscape in breast cancer (BC), irrespective of the HR-receptor status. The use of the agent is increasing, despite the finding that exposure to T-DXd increases the risk of interstitial lung disease (ILD), particularly in BC patients. Although T-DXd-related ILD can be potentially severe and life-threatening, most low-grade cases can be treated safely using a multidisciplinary approach comprising early and accurate diagnosis, effective management, close monitoring, and the prompt administration of steroids. Additionally, increasing patients' education on ILD symptoms ensures close attention and enables prompt reporting, enhancing patient outcomes. It is recommended that predictive biomarkers are assessed in patients with risk factors for developing ILD. Currently, diagnostic criteria comprise newly identified pulmonary opacities, the relation of symptom onset to medication initiation, and the exclusion of other causes of ILD. The general condition of patients is weakened during the management of ILD (BC progression and corticosteroid treatment). Consequently, BC chemotherapy might be attenuated. This highlights the importance of preventing (high-grade) ILD, especially since its use is expanded. Identifying high-risk patients, diagnosing, and customizing treatment is, however, challenging and additional information on patient selection is often not fully clarified. In this paper, we provide updated multidisciplinary clinical guidance for patient selection, proactive monitoring, early diagnosis, and effectively management of T-DXd-induced ILD in HER2-positive BC patients. We describe the risk factors for developing ILD, patients' characteristics of ILD, and the histopathological and radiographic characteristics of ILD, including real-world clinical practice reports. These recommendations provide a structured step-by-step approach for managing each suspected BC-related ILD grade.
Keywords: HER2-negative breast cancer; HER2-positive breast cancer; T-DXd-induced ILD; interstitial lung disease; multidisciplinary guidance; trastuzumab deruxtecan.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: AM received a research grant from Eli Lilly. Consulting fees from Eli Lilly, Astra-Zeneca, Seagen, Daiichi, Gilead. Support for attending meetings and/or travel from Roche, Novartis Gilead. Participation on a data safety monitoring board or advisory board: Eli Lilly, Astra-Zeneca, Gilead, Daiichi. MS received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from MSD, MERCK, Servier, GSK, Amgen, Astra-Zeneca. Participation on a Data Safety Monitoring Board or Advisory Board: Rottapharmbiotech, Advisory Board MSD, Advisory Board MERCK, Advisory Board Servier, Advisory Board GSK. EL received a grant or contract from ESMO. Consulting fees from MSD/Astra-Zeneca. Support for attending meetings and/or travel from ESMO. Participation on a data safety monitoring board or advisory board: Astra-Zeneca. BP received grants or contracts from EraPerMed 2019 and MSD. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis. Support for attending meetings and/or travel from ESMO and Eli Lilly. Leadership or fiduciary role in other board, society, committee or advocacy group: ESMO, POWG. CS received travel grants from Eli Lilly. ND received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from BMS, MSD, MERCK. All other authors have declared no conflicts of interest.
Figures


References
-
- Giaquinto A.N., Sung H., Miller K.D., et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524–541. - PubMed
-
- Tamura K., Tsurutani J., Takahashi S., et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):816–826. - PubMed
-
- GmbH. DSE . 2023. Enhertu 100 mg Powder for Concentrate for Solution for Infusion: EU Summary of Product Characteristics.
-
- Narayan P., Dilawari A., Osgood C., et al. US food and drug administration approval summary: fam-trastuzumab deruxtecan-nxki for human epidermal growth factor receptor 2-low unresectable or metastatic breast cancer. J Clin Oncol. 2023;41(11):2108–2116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

