Developing multispecies quorum-sensing modulators based on the Streptococcus mitis competence-stimulating peptide
- PMID: 37951305
- PMCID: PMC10714334
- DOI: 10.1016/j.jbc.2023.105448
Developing multispecies quorum-sensing modulators based on the Streptococcus mitis competence-stimulating peptide
Abstract
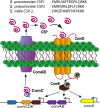
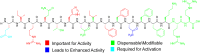
Bacteria utilize quorum sensing (QS) to coordinate many group behaviors. As such, QS has attracted significant attention as a potential mean to attenuate bacterial infectivity without introducing selective pressure for resistance development. Streptococcus mitis, a human commensal, acts as a genetic diversity reservoir for Streptococcus pneumoniae, a prevalent human pathogen. S. mitis possesses a typical comABCDE competence regulon QS circuitry; however, the competence-stimulating peptide (CSP) responsible for QS activation and the regulatory role of the competence regulon QS circuitry in S. mitis are yet to be explored. We set out to delineate the competence regulon QS circuitry in S. mitis, including confirming the identity of the native CSP signal, evaluating the molecular mechanism that governs CSP interactions with histidine kinase receptor ComD leading to ComD activation, and defining the regulatory roles of the competence regulon QS circuitry in initiating various S. mitis phenotypes. Our analysis revealed important structure-activity relationship insights of the CSP signal and facilitated the development of novel CSP-based QS modulators. Our analysis also revealed the involvement of the competence regulon in modulating competence development and biofilm formation. Furthermore, our analysis revealed that the native S. mitis CSP signal can modulate QS response in S. pneumoniae. Capitalizing on this crosstalk, we developed a multispecies QS modulator that activates both the pneumococcus ComD receptors and the S. mitis ComD-2 receptor with high potencies. The novel scaffolds identified herein can be utilized to evaluate the effects temporal QS modulation has on S. mitis as it inhabits its natural niche.
Keywords: Streptococcus mitis; Streptococcus pneumoniae; Structure-activity relationships; competence stimulating peptide (CSP); quorum sensing.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures






Similar articles
-
Elucidating the Role of the Competence Regulon Quorum Sensing Circuitry in Streptococcus cristatus.ACS Chem Biol. 2025 May 16;20(5):1123-1136. doi: 10.1021/acschembio.5c00180. Epub 2025 Apr 21. ACS Chem Biol. 2025. PMID: 40257361
-
Biological Evaluation of Native Streptococcal Competence Stimulating Peptides Reveal Potential Crosstalk Between Streptococcus mitis and Streptococcus pneumoniae and a New Scaffold for the Development of S. pneumoniae Quorum Sensing Modulators.RSC Chem Biol. 2020 Jun 1;1(2):60-67. doi: 10.1039/D0CB00012D. Epub 2020 May 29. RSC Chem Biol. 2020. PMID: 32905481 Free PMC article.
-
Structure-Activity Relationships of the Competence Stimulating Peptides (CSPs) in Streptococcus pneumoniae Reveal Motifs Critical for Intra-group and Cross-group ComD Receptor Activation.ACS Chem Biol. 2017 Apr 21;12(4):1141-1151. doi: 10.1021/acschembio.7b00007. Epub 2017 Mar 10. ACS Chem Biol. 2017. PMID: 28221753 Free PMC article.
-
Competence in Streptococcus pneumoniae and Close Commensal Relatives: Mechanisms and Implications.Front Cell Infect Microbiol. 2019 Apr 3;9:94. doi: 10.3389/fcimb.2019.00094. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31001492 Free PMC article. Review.
-
Quorum sensing and biofilm formation by Streptococcus mutans.Adv Exp Med Biol. 2008;631:178-88. doi: 10.1007/978-0-387-78885-2_12. Adv Exp Med Biol. 2008. PMID: 18792689 Review.
Cited by
-
Metabolic and Lipid Biomarkers for Pathogenic Algae, Fungi, Cyanobacteria, Mycobacteria, Gram-Positive Bacteria, and Gram-Negative Bacteria.Metabolites. 2024 Jul 6;14(7):378. doi: 10.3390/metabo14070378. Metabolites. 2024. PMID: 39057701 Free PMC article. Review.
-
Deciphering the Regulatory Role and Molecular Interactions That Drive the Competence Regulon Quorum Sensing Circuitry in Streptococcus constellatus.Biochemistry. 2025 Jul 15;64(14):3104-3113. doi: 10.1021/acs.biochem.5c00195. Epub 2025 Jun 23. Biochemistry. 2025. PMID: 40548891
-
Molecular mechanisms and applications of natural transformation in bacteria.Front Microbiol. 2025 Jun 24;16:1578813. doi: 10.3389/fmicb.2025.1578813. eCollection 2025. Front Microbiol. 2025. PMID: 40630188 Free PMC article. Review.
-
Elucidating the Role of the Competence Regulon Quorum Sensing Circuitry in Streptococcus cristatus.ACS Chem Biol. 2025 May 16;20(5):1123-1136. doi: 10.1021/acschembio.5c00180. Epub 2025 Apr 21. ACS Chem Biol. 2025. PMID: 40257361
References
-
- Milly T.A., Tal-Gan Y. Biological evaluation of native streptococcal competence stimulating peptides reveals potential crosstalk between Streptococcus mitis and Streptococcus pneumoniae and a new scaffold for the development of S. pneumoniae quorum sensing modulators. RSC Chem. Biol. 2020;1:60–67. - PMC - PubMed
-
- Huang S.S., Johnson K.M., Ray G.T., Wroe P., Lieu T.A., Moore M.R., et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–3412. - PubMed
-
- Mehr S., Wood N. Streptococcus pneumoniae--a review of carriage, infection, serotype replacement and vaccination. Paediatr. Respir. Rev. 2012;13:258–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

