Cross-alteration of murine skin and tick microbiome concomitant with pathogen transmission after Ixodes ricinus bite
- PMID: 37952001
- PMCID: PMC10638774
- DOI: 10.1186/s40168-023-01696-7
Cross-alteration of murine skin and tick microbiome concomitant with pathogen transmission after Ixodes ricinus bite
Abstract
Background: Ticks are major vectors of diseases affecting humans such as Lyme disease or domestic animals such as anaplasmosis. Cross-alteration of the vertebrate host skin microbiome and the tick microbiome may be essential during the process of tick feeding and for the mechanism of pathogen transmission. However, it has been poorly investigated.
Methods: We used mice bitten by field-collected ticks (nymphs and adult ticks) in different experimental conditions to investigate, by 16S rRNA gene metabarcoding, the impact of blood feeding on both the mouse skin microbiome and the tick microbiome. We also investigated by PCR and 16S rRNA gene metabarcoding, the diversity of microorganisms transmitted to the host during the process of tick bite at the skin interface and the dissemination of the pathogen in host tissues (blood, heart, and spleen).
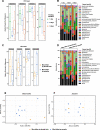
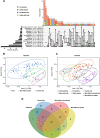
Results: Most of the commensal bacteria present in the skin of control mice were replaced during the blood-feeding process by bacteria originating from the ticks. The microbiome of the ticks was also impacted by the blood feeding. Several pathogens including tick-borne pathogens (Borrelia/Borreliella, Anaplasma, Neoehrlichia, Rickettsia) and opportunistic bacteria (Williamsia) were transmitted to the skin microbiome and some of them disseminated to the blood or spleen of the mice. In the different experiments of this study, skin microbiome alteration and Borrelia/Borreliella transmission were different depending on the tick stages (nymphs or adult female ticks).
Conclusions: Host skin microbiome at the bite site was deeply impacted by the tick bite, to an extent which suggests a role in the tick feeding, in the pathogen transmission, and a potentially important impact on the skin physiopathology. The diversified taxonomic profiles of the tick microbiome were also modified by the blood feeding. Video Abstract.
Keywords: 16S targeted sequencing; Anaplasma; Borrelia; Borreliella; Lyme disease; Metagenome; Metagenomic; Microbiota.
© 2023. The Author(s).
Conflict of interest statement
A.R., F.S. and B.L. are shareholders of Vaiomer.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

