COVID-19 screening in low resource settings using artificial intelligence for chest radiographs and point-of-care blood tests
- PMID: 37952026
- PMCID: PMC10640556
- DOI: 10.1038/s41598-023-46461-w
COVID-19 screening in low resource settings using artificial intelligence for chest radiographs and point-of-care blood tests
Abstract
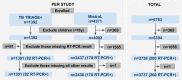
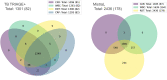
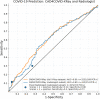
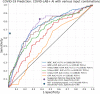
Artificial intelligence (AI) systems for detection of COVID-19 using chest X-Ray (CXR) imaging and point-of-care blood tests were applied to data from four low resource African settings. The performance of these systems to detect COVID-19 using various input data was analysed and compared with antigen-based rapid diagnostic tests. Participants were tested using the gold standard of RT-PCR test (nasopharyngeal swab) to determine whether they were infected with SARS-CoV-2. A total of 3737 (260 RT-PCR positive) participants were included. In our cohort, AI for CXR images was a poor predictor of COVID-19 (AUC = 0.60), since the majority of positive cases had mild symptoms and no visible pneumonia in the lungs. AI systems using differential white blood cell counts (WBC), or a combination of WBC and C-Reactive Protein (CRP) both achieved an AUC of 0.74 with a suggested optimal cut-off point at 83% sensitivity and 63% specificity. The antigen-RDT tests in this trial obtained 65% sensitivity at 98% specificity. This study is the first to validate AI tools for COVID-19 detection in an African setting. It demonstrates that screening for COVID-19 using AI with point-of-care blood tests is feasible and can operate at a higher sensitivity level than antigen testing.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

