Taming the perils of photosynthesis by eukaryotes: constraints on endosymbiotic evolution in aquatic ecosystems
- PMID: 37952050
- PMCID: PMC10640588
- DOI: 10.1038/s42003-023-05544-0
Taming the perils of photosynthesis by eukaryotes: constraints on endosymbiotic evolution in aquatic ecosystems
Abstract
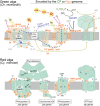
An ancestral eukaryote acquired photosynthesis by genetically integrating a cyanobacterial endosymbiont as the chloroplast. The chloroplast was then further integrated into many other eukaryotic lineages through secondary endosymbiotic events of unicellular eukaryotic algae. While photosynthesis enables autotrophy, it also generates reactive oxygen species that can cause oxidative stress. To mitigate the stress, photosynthetic eukaryotes employ various mechanisms, including regulating chloroplast light absorption and repairing or removing damaged chloroplasts by sensing light and photosynthetic status. Recent studies have shown that, besides algae and plants with innate chloroplasts, several lineages of numerous unicellular eukaryotes engage in acquired phototrophy by hosting algal endosymbionts or by transiently utilizing chloroplasts sequestrated from algal prey in aquatic ecosystems. In addition, it has become evident that unicellular organisms engaged in acquired phototrophy, as well as those that feed on algae, have also developed mechanisms to cope with photosynthetic oxidative stress. These mechanisms are limited but similar to those employed by algae and plants. Thus, there appear to be constraints on the evolution of those mechanisms, which likely began by incorporating photosynthetic cells before the establishment of chloroplasts by extending preexisting mechanisms to cope with oxidative stress originating from mitochondrial respiration and acquiring new mechanisms.
© 2023. The Author(s).
Conflict of interest statement
The author declares no competing interests.
Figures



References
-
- Stoecker DK, Johnson M, deVargas C, Not F. Acquired phototrophy in aquatic protists. Aquat. Micro. Ecol. 2009;57:279–310. doi: 10.3354/ame01340. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

