The Safety, Tolerability, Pharmacokinetics, and Clinical Efficacy of the NLRX1 agonist NX-13 in Active Ulcerative Colitis: Results of a Phase 1b Study
- PMID: 37952114
- PMCID: PMC11140628
- DOI: 10.1093/ecco-jcc/jjad192
The Safety, Tolerability, Pharmacokinetics, and Clinical Efficacy of the NLRX1 agonist NX-13 in Active Ulcerative Colitis: Results of a Phase 1b Study
Abstract
Background and aims: NX-13 activation of NLRX1 reduces intracellular reactive oxygen species and decreases inflammation in animal models of colitis. A phase 1a trial demonstrated a gut-selective pharmacokinetic profile with good tolerability. This phase Ib study aimed to evaluate the safety, tolerability, and pharmacokinetics of NX-13 in patients with active ulcerative colitis [UC].
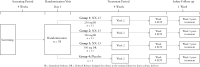
Methods: We conducted a multicentre, randomized, double-blind, placebo-controlled trial of NX-13 in patients with active UC. Patients with a Mayo Clinic Score of 4-10 were randomly assigned [3:3:3:1 ratio] to three NX-13 oral dose groups (250 mg immediate release [IR], 500 mg IR, or 500 mg delayed release [DR], or placebo) once daily for 4 weeks. Safety and pharmacokinetics were the primary and secondary objectives, respectively.
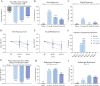
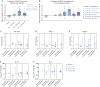
Results: Thirty-eight patients [11 females] were recruited and randomized to placebo [five], NX-13 250 mg IR [11], NX-13 500 mg IR [11], or NX-13 500 mg DR [11] and received at least one dose. There were no serious adverse events or deaths during the trial. One patient [500 mg DR, 1/11] withdrew due to worsening of UC and a second [500 mg IR, 1/11] on the last day of treatment after a panic attack associated with atrial fibrillation. In the efficacy population [36 patients], clinical improvement in rectal bleeding and stool frequency scores relative to placebo were seen as early as week 2 and endoscopic response was seen at week 4.
Conclusions: NX-13 was generally safe and well tolerated with early signs of rapid symptom and endoscopic improvement. This novel mechanism of action warrants further investigation. ClinicalTrials.gov: NCT04862741.
Keywords: NEXUS; NLRX1; NX-13; Randomized controlled clinical trial; immunometabolism; novel mechanism of action; phase 1b.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
BV has received grants from Abbvie, Biora Therapeutics, Landos, Pfizer, Sossei Heptares, and Takeda; speaker’s fees from Abbvie, Biogen, Bristol Myers Squibb, Celltrion, Chiesi, Falk, Ferring, Galapagos, Janssen, MSD, Pfizer, R-Biopharm, Takeda, Truvion, and Viatris; consultancy fees from Abbvie, Alimentiv, Applied Strategic, Atheneum, Biora Therapeutics, Bristol Myers Squibb, Galapagos, Guidepoint, Landos, Mylan, Inotrem, Ipsos, Janssen, Progenity, Sandoz, Sosei Heptares, Takeda, Tillots Pharma, and Viatris. SV has received grants from AbbVie, J&J, Pfizer, Takeda, and Galapagos; consulting and/or speaking fees from AbbVie, Abivax, AbolerIS Pharma, AgomAb, Alimentiv, Arena Pharmaceuticals, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Cytoki Pharma, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, GSK, Hospira, Imidomics, Janssen, J&J, Lilly, Materia Prima, MiroBio, Morphic, MrMHealth, Mundipharma, MSD, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance, Tillots Pharma AG, and Zealand Pharma. LPB has received fees from: AbbVie, Adacyte, Alimentiv, Alma Bio Therapeutics, Amgen, Applied Molecular Transport, Arena, Biogen, BMS, Celltrion, CONNECT Biopharm, Cytoki Pharma, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead, Gossamer Bio, GSK, HAC-Pharma, IAG Image Analysis, Index Pharmaceuticals, Inotrem, Janssen, Lilly, Medac, Mopac, Morphic, MSD, Norgine, Nordic Pharma, Novartis, OM Pharma, ONO Pharma, OSE Immunotherapeutics, Pandion Therapeutics, Par’Immune, Pfizer, Prometheus, Protagonist, Roche, Sanofi, Sandoz, Takeda, Theravance, Thermo Fisher, Tigenix, Tillots, Viatris, Vifor, Ysopia, Abivax, Samsung, Ventyx, Roivant, and Vectivbio. RAM is an employee and holds stock options in Landos Biopharma. BGF is a scientific advisory board member for AbbVie, Allergan, Amgen, AstraZeneca, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Elan, Biogen, Ferring, Genentech–Roche, Janssen–Johnson & Johnson, Merck, Millennium, Nestlé, Novo Nordisk, Novartis, Pfizer, Prometheus, Protagonist, Receptos, Salix, Sigmoid Pharma, Takeda, Teva, TiGenix, Tillotts Pharma, and UCB Pharma; consulting fees from AbbVie, Actogenix, Akros, Albireo Pharma, Allergan, Amgen, AstraZeneca, Avaxia Biologics, Avir Pharma, Axcan, Baxter Healthcare, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan–Biogen, EnGene, Ferring, Genentech–Roche, GiCare Pharma, Gilead Sciences, Given Imaging, GlaxoSmithKline, Ironwood, Janssen Biotech–Centocor, Janssen–Johnson & Johnson, Kyowa Hakko Kirin, Eli Lilly, Merck, Mesoblast Pharma, Millennium, Nestlé, Novo Nordisk, Novartis, Pfizer, Prometheus, Protagonist, Receptos Salix, Sanofi, Shire, Sigmoid Pharma, Synergy Pharma, Takeda, Teva, TiGenix, Tillotts Pharma, UCB Pharma, Vertex, VHsquared, Wyeth, Zealand, and Zygenia; lecture fees from AbbVie, Janssen–Johnson & Johnson, Takeda, and UCB Pharma; and grant support from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Janssen Biotech–Centocor, Janssen–Johnson & Johnson, Pfizer, Receptos, Sanofi, and Takeda; he is the Senior Scientific Officer of Alimentiv Inc. JFC reports receiving research grants from AbbVie, Janssen Pharmaceuticals, Takeda, and Bristol Myers Squibb; payment for lectures from AbbVie and Takeda; and consulting fees from AbbVie, Amgen, AnaptysBio, Allergan, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Celltrion, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Glaxo Smith Kline, Genentech [Roche], Janssen Pharmaceuticals, Kaleido Biosciences, Immunic, Iterative Scopes, Merck, Landos, Microba Life Science, Novartis, Otsuka Pharmaceutical, Pfizer, Protagonist Therapeutics, Sanofi, Takeda, TiGenix, and Vifor; he also holds stock options in Intestinal Biotech Development. BS has received grant support from Pfizer [to institution]; served as a consultant [served as a representative of Charité] for AbbVie, Arena, BMS, Boehringer, Celgene, Endpoint Health, Falk, Galapagos, Janssen, Landos, Lilly, Pfizer, PredictImmune, and Takeda; and has received speaker’s fees [served as a representative of Charité] from AbbVie, CED Service GmbH, Falk, Ferring, Galapagos, Janssen, Lilly, Pfizer, and Takeda; support for attending meetings/travel from Falk; and served as president of ECCO. FR was consultant to AbbVie, Adnovate, Agomab, Allergan, Arena, Astra-Zeneca, Boehringer-Ingelheim, Celgene/BMS, CDISC, Celsius, Cowen, Ferring, Galapagos, Galmed, Genentech, Gilead, Gossamer, Granite, Guidepoint, Helmsley, Horizon Therapeutics, Image Analysis Limited, Index Pharma, Landos, Jannsen, Koutif, Mestag, Metacrine, Mopac, Morphic, Organovo, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Sanofi, Surmodics, Surrozen, Takeda, Techlab, Teva, Theravance, Thetis, UCB, Ysios, and 89Bio. SS has received personal fees from AbbVie, Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Dr Falk Pharma, Ferring, Fresenius, Galapagos, Genentech, GSK, Gilead, I-MAB Biopharma, Janssen, Lilly, Merck, Novartis-Sandoz, Pfizer, Protagonist, Takeda, and Theravance. AY reports consulting fees from Takeda, Prometheus Labs, Arena Pharmaceuticals, and Bristol Myers Squibb; and speaker bureau fees from Bristol Myers Squibb. RP has served as a consultant for Abbott, AbbVie, Alimentiv [formerly Robarts], Amgen, Arena Pharmaceuticals, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Fresenius Kabi, Genentech, Gilead Sciences, Glaxo-Smith Kline, JAMP Bio, Janssen, Merck, Mylan, Novartis, Oppilan Pharma, Organon, Pandion Pharma, Pendopharm, Pfizer, Progenity, Protagonist Therapeutics, Roche, Sandoz, Satisfai Health, Shire, Sublimity Therapeutics, Takeda Pharmaceuticals, Theravance Biopharma, Trellus, Viatris, and UCB; and has received speaking fees from AbbVie, Amgen, Arena Pharmaceuticals, Bristol-Myers Squibb, Celgene, Eli Lilly, Ferring, Fresenius Kabi, Gilead Sciences, Janssen, Merck, Organon, Pfizer, Roche, Sandoz, Shire, and Takeda Pharmaceuticals. MCD reports consulting fees from AbbVie, Arena, Bristol Myers Squibb, Celgene, Eli Lilly, Genentech, Gilead, Janssen, Pfizer, Prometheus Labs, and Takeda; and is founder and a shareholder of Trellus Health. SL is a consultant for and holds stock in Landos Biopharma. FC is an employee and holds stock options in Landos Biopharma. SD reports consultancy fees from AbbVie, Alimentiv, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB Inc., and Vifor; he reports lecture fees from Abbvie, Amgen, Ferring Pharmaceuticals Inc., Gilead, Janssen, Mylan, Pfizer, and Takeda.
Figures


References
-
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. - PubMed
-
- Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011;60:780–7. - PubMed
-
- Sandborn WJ, Feagan BG, Marano C, et al. ; PURSUIT-SC Study Group. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85–95; quiz e14. - PubMed
-
- Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. - PubMed
-
- Sands BE, Sandborn WJ, Panaccione R, et al. ; UNIFI Study Group. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. - PubMed

