Is underutilization of adjuvant therapy in resected non-small-cell lung cancer associated with socioeconomic disparities?
- PMID: 37952179
- PMCID: PMC11007729
- DOI: 10.1093/ejcts/ezad383
Is underutilization of adjuvant therapy in resected non-small-cell lung cancer associated with socioeconomic disparities?
Erratum in
-
Corrigendum to: Minimally invasive surgery for clinical T4 non-small-cell lung cancer: national trends and outcomes; Is underutilization of adjuvant therapy in resected non-small-cell lung cancer associated with socioeconomic disparities?; and Sublobar resection is associated with less lymph nodes examined and lower delivery of adjuvant therapy in patients with 1.5- to 2.0-cm clinical IA2 non-small-cell lung cancer: a retrospective cohort study.Eur J Cardiothorac Surg. 2024 May 3;65(5):ezae203. doi: 10.1093/ejcts/ezae203. Eur J Cardiothorac Surg. 2024. PMID: 38783681 Free PMC article. No abstract available.
Abstract
Objectives: Although adjuvant systemic therapy (AT) has demonstrated improved survival in patients with resected non-small-cell lung cancer (NSCLC), it remains underutilized. Recent trials demonstrating improved outcomes with adjuvant immunotherapy and targeted treatment imply that low uptake of systemic therapy in at-risk populations may widen existing outcome gaps. We, therefore, sought to determine factors associated with the underutilization of AT.
Methods: The National Cancer Database (2010-2018) was queried for patients with completely resected stage II-IIIA NSCLC and stratified based on the receipt of AT. Logistic regression was used to identify factors associated with AT delivery. The Kaplan-Meier method was applied to estimate survival after propensity-matching to adjust for confounders.
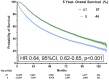
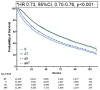
Results: Of 37 571 eligible patients, only 20 616 (54.9%) received AT. While AT rates increased over time, multivariable analysis showed that older age [adjusted odds ratio (aOR) 0.45, 95% confidence interval (CI) 0.43-0.47], male sex (aOR 0.88, 95% CI 0.85-0.93) and multiple comorbidities (aOR 0.86, 95% CI: 0.81-0.91) were associated with decreased AT. Socioeconomic factors were additionally associated with underutilization, including public insurance (aOR 0.70, 95% CI: 0.66-0.74), lower education indicators (aOR 0.93, 95% CI: 0.88-0.97) and living more than 10 miles from a treatment facility (aOR 0.89, 95% CI: 0.85-0.93). After propensity matching, receipt of adjuvant therapy was associated with improved overall survival (median 76.35 vs 47.57 months, P ≤ 0.001).
Conclusions: AT underutilization in patients with resected stage II-III NSCLC is associated with patient, institutional and socioeconomic factors. It is critical to implement measures to address these inequities, especially in light of newer adjuvant immunotherapy and targeted therapy treatment options which are expected to improve survival.
Keywords: Adjuvant therapy; Disparities; Immunotherapy; Non-small-cell lung cancer; Systemic therapy.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery. All rights reserved.
Conflict of interest statement
Conflict of interest: Brendon Stiles: Medtronic, AstraZeneca, Genentech, Pfizer, Arcus Biosciences, Merck, Bristol Myers Squib, BMS Foundation, Galvanize Therapeutics and the Lung Cancer Research Foundation. The rest of the authors have no conflicts of interest to disclose. Neel P. Chudgar: AstraZeneca.
Figures



Comment in
-
A commentary on lung cancer healthcare disparities.Eur J Cardiothorac Surg. 2023 Dec 1;64(6):ezad401. doi: 10.1093/ejcts/ezad401. Eur J Cardiothorac Surg. 2023. PMID: 38085179 No abstract available.
References
-
- Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet 2021;398:535–54. - PubMed
-
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A et al. NCCN Guidelines® Insights: non-small cell lung cancer, version 2.2023: featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw 2023;21:340–50. - PubMed
-
- Kidane B, Bott M, Spicer J, Backhus L, Chaft J, Chudgar N et al.; Expert Consensus Panel. The American Association for Thoracic Surgery (AATS) 2023 Expert Consensus Document: staging and multidisciplinary management of patients with early-stage non–small cell lung cancer. J Thorac Cardiovasc Surg 2023;166:637–54. - PubMed
-
- Pignon J-P, Tribodet H, Scagliotti GV, Douillard J-Y, Shepherd FA, Stephens RJ et al.; LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

