Effects of Semaglutide on Symptoms, Function, and Quality of Life in Patients With Heart Failure With Preserved Ejection Fraction and Obesity: A Prespecified Analysis of the STEP-HFpEF Trial
- PMID: 37952180
- PMCID: PMC10782938
- DOI: 10.1161/CIRCULATIONAHA.123.067505
Effects of Semaglutide on Symptoms, Function, and Quality of Life in Patients With Heart Failure With Preserved Ejection Fraction and Obesity: A Prespecified Analysis of the STEP-HFpEF Trial
Abstract
Background: Patients with heart failure (HF) with preserved ejection fraction (HFpEF) and obesity experience a high burden of symptoms and functional impairment, and a poor quality of life. In the STEP-HFpEF trial (Research Study to Investigate How Well Semaglutide Works in People Living With Heart Failure and Obesity), once-weekly semaglutide 2.4 mg improved symptoms, physical limitations, and exercise function, and reduced inflammation and body weight. This prespecified analysis investigated the effects of semaglutide on the primary and confirmatory secondary end points across the range of the Kansas City Cardiomyopathy Questionnaire (KCCQ) scores at baseline and on all key summary and individual KCCQ domains.
Methods: STEP-HFpEF randomly assigned 529 participants with symptomatic HF, an ejection fraction of ≥45%, and a body mass index of ≥30 kg/m2 to once-weekly semaglutide 2.4 mg or placebo for 52 weeks. Dual primary end points change in KCCQ-Clinical Summary Score (CSS) and body weight. Confirmatory secondary end points included change in 6-minute walk distance, a hierarchical composite end point (death, HF events, and change in KCCQ-CSS and 6-minute walk distance) and change in C-reactive protein. Patients were stratified by KCCQ-CSS tertiles at baseline. Semaglutide effects on the primary, confirmatory secondary, and select exploratory end points (N-terminal pro-brain natriuretic peptide) were examined across these subgroups. Semaglutide effects on additional KCCQ domains (Total Symptom Score [including symptom burden and frequency], Physical Limitations Score, Social Limitations Score, Quality of Life Score, and Overall Summary Score) were also evaluated.
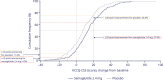
Results: Baseline median KCCQ-CSS across tertiles was 37, 59, and 77 points, respectively. Semaglutide consistently improved primary end points across KCCQ tertiles 1 to 3 (estimated treatment differences [95% CI]: for KCCQ-CSS, 10.7 [5.4 to 16.1], 8.1 [2.7 to 13.4], and 4.6 [-0.6 to 9.9] points; for body weight, -11 [-13.2 to -8.8], -9.4 [-11.5 to -7.2], and -11.8 [-14.0 to -9.6], respectively; Pinteraction=0.28 and 0.29, respectively); the same was observed for confirmatory secondary and exploratory end points (Pinteraction>0.1 for all). Semaglutide-treated patients experienced improvements in all key KCCQ domains (estimated treatment differences, 6.7-9.6 points across domains; P≤0.001 for all). Greater proportion of semaglutide-treated versus placebo-treated patients experienced at least 5-, 10-, 15-, and 20-point improvements in all KCCQ domains (odds ratios, 1.6-2.9 across domains; P<0.05 for all).
Conclusions: In patients with HFpEF and obesity, semaglutide produced large improvements in HF-related symptoms, physical limitations, exercise function, inflammation, body weight, and N-terminal pro-brain natriuretic peptide, regardless of baseline health status. The benefits of semaglutide extended to all key KCCQ domains.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04788511.
Keywords: health status; heart failure, diastolic; obesity; quality of life; semaglutide; weight loss.
Conflict of interest statement
Figures





Comment in
-
In HFpEF with obesity, semaglutide improved health status and weight loss at 52 wk, regardless of initial health status.Ann Intern Med. 2024 May;177(5):JC56. doi: 10.7326/J24-0025. Epub 2024 May 7. Ann Intern Med. 2024. PMID: 38710083
References
-
- Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. 2023;329:827–838. doi: 10.1001/jama.2023.2020 - PubMed
-
- Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC Scientific Statement. J Am Coll Cardiol. 2023;81:1810–1834. doi: 10.1016/j.jacc.2023.01.049 - PubMed
-
- Morgen CS, Haase CL, Oral TK, Schnecke V, Varbo A, Borlaug BA. Obesity, cardiorenal comorbidities, and risk of hospitalization in patients with heart failure with preserved ejection fraction. Mayo Clin Proc. 2023;98:1458–1468. doi: 10.1016/j.mayocp.2023.07.008 - PubMed
-
- Dalos D, Mascherbauer J, Zotter-Tufaro C, Duca F, Kammerlander AA, Aschauer S, Bonderman D. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:189–199. doi: 10.1016/j.jacc.2016.04.052 - PubMed
-
- Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol. 2016;68:200–203. doi: 10.1016/j.jacc.2016.05.019 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 HL128526/HL/NHLBI NIH HHS/United States
- R01 HL162828/HL/NHLBI NIH HHS/United States
- U01 HL160226/HL/NHLBI NIH HHS/United States
- U54 HL160273/HL/NHLBI NIH HHS/United States
- R01 HL107577/HL/NHLBI NIH HHS/United States
- R01 HL127028/HL/NHLBI NIH HHS/United States
- R01 HL140731/HL/NHLBI NIH HHS/United States
- R01 HL149423/HL/NHLBI NIH HHS/United States
- U01 AG076928/AG/NIA NIH HHS/United States
- R01 AG078153/AG/NIA NIH HHS/United States
- R01 AG045551/AG/NIA NIH HHS/United States
- R01 AG018915/AG/NIA NIH HHS/United States
- P30 AG021332/AG/NIA NIH HHS/United States
- U24 AG059624/AG/NIA NIH HHS/United States
- U01 HL160272/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

