Clonal haematopoiesis of indeterminate potential predicts incident cardiac arrhythmias
- PMID: 37952204
- PMCID: PMC10919923
- DOI: 10.1093/eurheartj/ehad670
Clonal haematopoiesis of indeterminate potential predicts incident cardiac arrhythmias
Abstract
Background and aims: Clonal haematopoiesis of indeterminate potential (CHIP), the age-related expansion of blood cells with preleukemic mutations, is associated with atherosclerotic cardiovascular disease and heart failure. This study aimed to test the association of CHIP with new-onset arrhythmias.
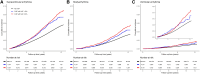
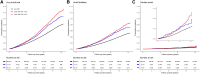
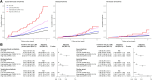
Methods: UK Biobank participants without prevalent arrhythmias were included. Co-primary study outcomes were supraventricular arrhythmias, bradyarrhythmias, and ventricular arrhythmias. Secondary outcomes were cardiac arrest, atrial fibrillation, and any arrhythmia. Associations of any CHIP [variant allele fraction (VAF) ≥ 2%], large CHIP (VAF ≥10%), and gene-specific CHIP subtypes with incident arrhythmias were evaluated using multivariable-adjusted Cox regression. Associations of CHIP with myocardial interstitial fibrosis [T1 measured using cardiac magnetic resonance (CMR)] were also tested.
Results: This study included 410 702 participants [CHIP: n = 13 892 (3.4%); large CHIP: n = 9191 (2.2%)]. Any and large CHIP were associated with multi-variable-adjusted hazard ratios of 1.11 [95% confidence interval (CI) 1.04-1.18; P = .001] and 1.13 (95% CI 1.05-1.22; P = .001) for supraventricular arrhythmias, 1.09 (95% CI 1.01-1.19; P = .031) and 1.13 (95% CI 1.03-1.25; P = .011) for bradyarrhythmias, and 1.16 (95% CI, 1.00-1.34; P = .049) and 1.22 (95% CI 1.03-1.45; P = .021) for ventricular arrhythmias, respectively. Associations were independent of coronary artery disease and heart failure. Associations were also heterogeneous across arrhythmia subtypes and strongest for cardiac arrest. Gene-specific analyses revealed an increased risk of arrhythmias across driver genes other than DNMT3A. Large CHIP was associated with 1.31-fold odds (95% CI 1.07-1.59; P = .009) of being in the top quintile of myocardial fibrosis by CMR.
Conclusions: CHIP may represent a novel risk factor for incident arrhythmias, indicating a potential target for modulation towards arrhythmia prevention and treatment.
Keywords: Aging; Arrhythmia; Atrial fibrillation; Cardiac arrest; Genomics; Prevention.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NAM, et al. . 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013;61:e6–75. 10.1016/j.jacc.2012.11.007 - DOI - PubMed
-
- Zöga Diederichsen S, Xing YL, Frodi DM, Kongebro EK, Haugan KJ, Graff C, et al. . Prevalence and prognostic significance of bradyarrhythmias in patients screened for atrial fibrillation vs usual care: post hoc analysis of the LOOP randomized clinical trial. JAMA Cardiol 2023;8:326–34. 10.1001/jamacardio.2022.5526 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

