The MAGIC trial: a pragmatic, multicentre, parallel, noninferiority, randomised trial of melatonin versus midazolam in the premedication of anxious children attending for elective surgery under general anaesthesia
- PMID: 37953202
- PMCID: PMC10797512
- DOI: 10.1016/j.bja.2023.10.011
The MAGIC trial: a pragmatic, multicentre, parallel, noninferiority, randomised trial of melatonin versus midazolam in the premedication of anxious children attending for elective surgery under general anaesthesia
Abstract
Background: Child anxiety before general anaesthesia and surgery is common. Midazolam is a commonly used premedication to address this. Melatonin is an alternative anxiolytic, however trials evaluating its efficacy in children have delivered conflicting results.
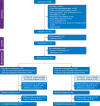
Methods: This multicentre, double-blind randomised trial was performed in 20 UK NHS Trusts. A sample size of 624 was required to declare noninferiority of melatonin. Anxious children, awaiting day case elective surgery under general anaesthesia, were randomly assigned 1:1 to midazolam or melatonin premedication (0.5 mg kg-1, maximum 20 mg) 30 min before transfer to the operating room. The primary outcome was the modified Yale Preoperative Anxiety Scale-Short Form (mYPAS-SF). Secondary outcomes included safety. Results are presented as n (%) and adjusted mean differences with 95% confidence intervals.
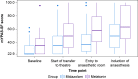
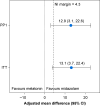
Results: The trial was stopped prematurely (n=110; 55 per group) because of recruitment futility. Participants had a median age of 7 (6-10) yr, and 57 (52%) were female. Intention-to-treat and per-protocol modified Yale Preoperative Anxiety Scale-Short Form analyses showed adjusted mean differences of 13.1 (3.7-22.4) and 12.9 (3.1-22.6), respectively, in favour of midazolam. The upper 95% confidence interval limits exceeded the predefined margin of 4.3 in both cases, whereas the lower 95% confidence interval excluded zero, indicating that melatonin was inferior to midazolam, with a difference considered to be clinically relevant. No serious adverse events were seen in either arm.
Conclusion: Melatonin was less effective than midazolam at reducing preoperative anxiety in children, although the early termination of the trial increases the likelihood of bias.
Clinical trial registration: ISRCTN registry: ISRCTN18296119.
Keywords: general anaesthesia; melatonin; midazolam; paediatric anxiety; perioperative care; premedication.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Sury M.R., Arumainathan R., Belhaj A.M., MacG Palmer J.H., Cook T.M., Pandit J.J. The state of UK pediatric anesthesia: a survey of National Health Service activity. Paediatr Anaesth. 2015;25:1085–1092. - PubMed
-
- Deery C, Bolt R, Papaioannou D, et al. The MAGIC trial (Melatonin for Anxiety prior to General anaesthesia In Children): a multicentre, parallel randomised controlled trial of melatonin versus midazolam in the premedication of anxious children attending for elective surgery under general anaesthesia. 2023. The University of Sheffield. Workflow. Available from: 10.15131/shef.data.22220884.v1 (accessed 23 March 2023) - DOI
-
- Kocherov S., Hen Y., Jaworowski S., et al. Medical clowns reduce pre-operative anxiety, post-operative pain and medical costs in children undergoing outpatient penile surgery: a randomised controlled trial. J Paediatr Child Health. 2016;52:877–881. - PubMed
-
- Arai Y.C., Kandatsu N., Ito H., et al. Induction and emergence behavior of children undergoing general anesthesia correlates with maternal salivary amylase activity before the anesthesia. Acta Anaesthesiol Scand. 2008;52:285–288. - PubMed

