TATDN2 resolution of R-loops is required for survival of BRCA1-mutant cancer cells
- PMID: 37953292
- PMCID: PMC10711561
- DOI: 10.1093/nar/gkad952
TATDN2 resolution of R-loops is required for survival of BRCA1-mutant cancer cells
Abstract
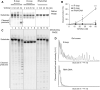
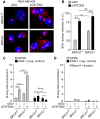
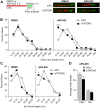
BRCA1-deficient cells have increased IRE1 RNase, which degrades multiple microRNAs. Reconstituting expression of one of these, miR-4638-5p, resulted in synthetic lethality in BRCA1-deficient cancer cells. We found that miR-4638-5p represses expression of TATDN2, a poorly characterized member of the TATD nuclease family. We discovered that human TATDN2 has RNA 3' exonuclease and endonuclease activity on double-stranded hairpin RNA structures. Given the cleavage of hairpin RNA by TATDN2, and that BRCA1-deficient cells have difficulty resolving R-loops, we tested whether TATDN2 could resolve R-loops. Using in vitro biochemical reconstitution assays, we found TATDN2 bound to R-loops and degraded the RNA strand but not DNA of multiple forms of R-loops in vitro in a Mg2+-dependent manner. Mutations in amino acids E593 and E705 predicted by Alphafold-2 to chelate an essential Mg2+ cation completely abrogated this R-loop resolution activity. Depleting TATDN2 increased cellular R-loops, DNA damage and chromosomal instability. Loss of TATDN2 resulted in poor replication fork progression in the presence of increased R-loops. Significantly, we found that TATDN2 is essential for survival of BRCA1-deficient cancer cells, but much less so for cognate BRCA1-repleted cancer cells. Thus, we propose that TATDN2 is a novel target for therapy of BRCA1-deficient cancers.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



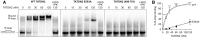
References
-
- Singh D., Rahi A., Kumari R., Gupta V., Gautam G., Aggarwal S., Rehan M., Bhatnagar R.. Computational and mutational analysis of TatD DNase of Bacillus anthracis. J. Cell. Biochem. 2019; 120:11318–11330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

