Trends in Outpatient Influenza Antiviral Use Among Children and Adolescents in the United States
- PMID: 37953658
- PMCID: PMC10681853
- DOI: 10.1542/peds.2023-061960
Trends in Outpatient Influenza Antiviral Use Among Children and Adolescents in the United States
Abstract
Background: Influenza antivirals improve outcomes in children with duration of symptoms <2 days and those at high risk for influenza complications. Real-world prescribing of influenza antivirals in the pediatric population is unknown.
Methods: We performed a cross-sectional study of outpatient and emergency department prescription claims in individuals <18 years of age included in the IBM Marketscan Commercial Claims and Encounters Database between July 1, 2010 and June 30, 2019. Influenza antiviral use was defined as any dispensing of oseltamivir, baloxavir, or zanamivir. The primary outcome was the rate of antiviral dispensing per 1000 enrolled children. Secondary outcomes included antiviral dispensing per 1000 influenza diagnoses and inflation-adjusted costs of antiviral agents. Outcomes were calculated and stratified by age, acute versus prophylactic treatment, influenza season, and geographic region.
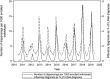
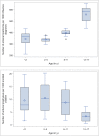
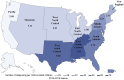
Results: The analysis included 1 416 764 unique antiviral dispensings between 2010 and 2019. Oseltamivir was the most frequently prescribed antiviral (99.8%). Dispensing rates ranged from 4.4 to 48.6 per 1000 enrolled children. Treatment rates were highest among older children (12-17 years of age), during the 2017 to 2018 influenza season, and in the East South Central region. Guideline-concordant antiviral use among young children (<2 years of age) at a high risk of influenza complications was low (<40%). The inflation-adjusted cost for prescriptions was $208 458 979, and the median cost ranged from $111 to $151.
Conclusions: There is wide variability and underuse associated with influenza antiviral use in children. These findings reveal opportunities for improvement in the prevention and treatment of influenza in children.
Copyright © 2023 by the American Academy of Pediatrics.
Conflict of interest statement
Figures
Comment in
-
Influenza Antivirals in Pediatrics: Why Aren't We Using All the Available Tools?Pediatrics. 2023 Dec 1;152(6):e2023063481. doi: 10.1542/peds.2023-063481. Pediatrics. 2023. PMID: 37953646 No abstract available.
References
-
- Somes MP, Turner RM, Dwyer LJ, Newall AT. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: a systematic review and meta-analysis. Vaccine. 2018;36(23):3199–3207 - PubMed
-
- Blyth CC, Macartney KK, McRae J, et al. ; Paediatric Active Enhanced Disease Surveillance (PAEDS); Influenza Complications Alert Network (FluCAN) Collaboration. Influenza epidemiology, vaccine coverage and vaccine effectiveness in children admitted to sentinel Australian hospitals in 2017: results from the PAEDS-FluCAN Collaboration. Clin Infect Dis. 2019;68(6):940–948 - PubMed
-
- Burton C, Vaudry W, Moore D, et al. ; IMPACT investigators. Burden of seasonal influenza in children with neurodevelopmental conditions. Pediatr Infect Dis J. 2014;33(7):710–714 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

