Hydrogel-Embedded Precision-Cut Lung Slices Model Lung Cancer Premalignancy Ex Vivo
- PMID: 37953708
- PMCID: PMC10872976
- DOI: 10.1002/adhm.202302246
Hydrogel-Embedded Precision-Cut Lung Slices Model Lung Cancer Premalignancy Ex Vivo
Abstract
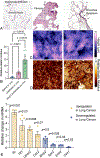
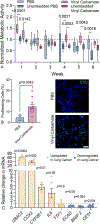
Lung cancer is the leading global cause of cancer-related deaths. Although smoking cessation is the best prevention, 50% of lung cancer diagnoses occur in people who have quit smoking. Research into treatment options for high-risk patients is constrained to rodent models, which are time-consuming, expensive, and require large cohorts. Embedding precision-cut lung slices (PCLS) within an engineered hydrogel and exposing this tissue to vinyl carbamate, a carcinogen from cigarette smoke, creates an in vitro model of lung cancer premalignancy. Hydrogel formulations are selected to promote early lung cancer cellular phenotypes and extend PCLS viability to six weeks. Hydrogel-embedded PCLS are exposed to vinyl carbamate, which induces adenocarcinoma in mice. Analysis of proliferation, gene expression, histology, tissue stiffness, and cellular content after six weeks reveals that vinyl carbamate induces premalignant lesions with a mixed adenoma/squamous phenotype. Putative chemoprevention agents diffuse through the hydrogel and induce tissue-level changes. The design parameters selected using murine tissue are validated with hydrogel-embedded human PCLS and results show increased proliferation and premalignant lesion gene expression patterns. This tissue-engineered model of human lung cancer premalignancy is the foundation for more sophisticated ex vivo models that enable the study of carcinogenesis and chemoprevention strategies.
Keywords: biomaterials; hydrogels; in vitro models; lung cancer; precision-cut lung slices; premalignancy; tissue engineering.
© 2023 Wiley-VCH GmbH.
Figures





References
-
- Robert P, ‘Conquering lung cancer: current status and prospects for the future’, Pulmonology 2020, 26. - PubMed
-
- Mikhael PG, Wohlwend J, Yala A, Karstens L, Xiang J, Takigami AK, Bourgouin PP, Chan P, Mrah S, Amayri W, Juan Y-H, Yang C-T, Wan Y-L, Lin G, Sequist LV, Fintelmann FJ, Barzilay R, ‘Sybil: A Validated Deep Learning Model to Predict Future Lung Cancer Risk From a Single Low-Dose Chest Computed Tomography’, Journal of Clinical Oncology 2023, JCO.22.01345. - PMC - PubMed
-
- Siegel RL, Miller KD, Wagle NS, Jemal A, ‘Cancer statistics, 2023’, CA Cancer J. Clin. 2023, 73, 17–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

