Assessment of white matter hyperintensity severity using multimodal magnetic resonance imaging
- PMID: 37953840
- PMCID: PMC10636521
- DOI: 10.1093/braincomms/fcad279
Assessment of white matter hyperintensity severity using multimodal magnetic resonance imaging
Abstract
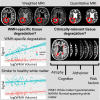
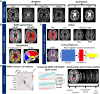
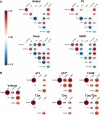
White matter hyperintensities are radiological abnormalities reflecting cerebrovascular dysfunction detectable using MRI. White matter hyperintensities are often present in individuals at the later stages of the lifespan and in prodromal stages in the Alzheimer's disease spectrum. Tissue alterations underlying white matter hyperintensities may include demyelination, inflammation and oedema, but these are highly variable by neuroanatomical location and between individuals. There is a crucial need to characterize these white matter hyperintensity tissue alterations in vivo to improve prognosis and, potentially, treatment outcomes. How different MRI measure(s) of tissue microstructure capture clinically-relevant white matter hyperintensity tissue damage is currently unknown. Here, we compared six MRI signal measures sampled within white matter hyperintensities and their associations with multiple clinically-relevant outcomes, consisting of global and cortical brain morphometry, cognitive function, diagnostic and demographic differences and cardiovascular risk factors. We used cross-sectional data from 118 participants: healthy controls (n = 30), individuals at high risk for Alzheimer's disease due to familial history (n = 47), mild cognitive impairment (n = 32) and clinical Alzheimer's disease dementia (n = 9). We sampled the median signal within white matter hyperintensities on weighted MRI images [T1-weighted (T1w), T2-weighted (T2w), T1w/T2w ratio, fluid-attenuated inversion recovery (FLAIR)] as well as the relaxation times from quantitative T1 (qT1) and T2* (qT2*) images. qT2* and fluid-attenuated inversion recovery signals within white matter hyperintensities displayed different age- and disease-related trends compared to normal-appearing white matter signals, suggesting sensitivity to white matter hyperintensity-specific tissue deterioration. Further, white matter hyperintensity qT2*, particularly in periventricular and occipital white matter regions, was consistently associated with all types of clinically-relevant outcomes in both univariate and multivariate analyses and across two parcellation schemes. qT1 and fluid-attenuated inversion recovery measures showed consistent clinical relationships in multivariate but not univariate analyses, while T1w, T2w and T1w/T2w ratio measures were not consistently associated with clinical variables. We observed that the qT2* signal was sensitive to clinically-relevant microstructural tissue alterations specific to white matter hyperintensities. Our results suggest that combining volumetric and signal measures of white matter hyperintensity should be considered to fully characterize the severity of white matter hyperintensities in vivo. These findings may have implications in determining the reversibility of white matter hyperintensities and the potential efficacy of cardio- and cerebrovascular treatments.
Keywords: cerebrovascular disease; dementia; microstructure; relaxometry; small vessel disease.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures



References
-
- Bos D, Wolters FJ, Darweesh SKL, et al. Cerebral small vessel disease and the risk of dementia: A systematic review and meta-analysis of population-based evidence. Alzheimers Dement. 2018;14(11):1482–1492. - PubMed
LinkOut - more resources
Full Text Sources
