Interictal magnetoencephalography abnormalities to guide intracranial electrode implantation and predict surgical outcome
- PMID: 37953844
- PMCID: PMC10636564
- DOI: 10.1093/braincomms/fcad292
Interictal magnetoencephalography abnormalities to guide intracranial electrode implantation and predict surgical outcome
Abstract
Intracranial EEG is the gold standard technique for epileptogenic zone localization but requires a preconceived hypothesis of the location of the epileptogenic tissue. This placement is guided by qualitative interpretations of seizure semiology, MRI, EEG and other imaging modalities, such as magnetoencephalography. Quantitative abnormality mapping using magnetoencephalography has recently been shown to have potential clinical value. We hypothesized that if quantifiable magnetoencephalography abnormalities were sampled by intracranial EEG, then patients' post-resection seizure outcome may be better. Thirty-two individuals with refractory neocortical epilepsy underwent magnetoencephalography and subsequent intracranial EEG recordings as part of presurgical evaluation. Eyes-closed resting-state interictal magnetoencephalography band power abnormality maps were derived from 70 healthy controls as a normative baseline. Magnetoencephalography abnormality maps were compared to intracranial EEG electrode implantation, with the spatial overlap of intracranial EEG electrode placement and cerebral magnetoencephalography abnormalities recorded. Finally, we assessed if the implantation of electrodes in abnormal tissue and subsequent resection of the strongest abnormalities determined by magnetoencephalography and intracranial EEG corresponded to surgical success. We used the area under the receiver operating characteristic curve as a measure of effect size. Intracranial electrodes were implanted in brain tissue with the most abnormal magnetoencephalography findings-in individuals that were seizure-free postoperatively (T = 3.9, P = 0.001) but not in those who did not become seizure-free. The overlap between magnetoencephalography abnormalities and electrode placement distinguished surgical outcome groups moderately well (area under the receiver operating characteristic curve = 0.68). In isolation, the resection of the strongest abnormalities as defined by magnetoencephalography and intracranial EEG separated surgical outcome groups well, area under the receiver operating characteristic curve = 0.71 and area under the receiver operating characteristic curve = 0.74, respectively. A model incorporating all three features separated surgical outcome groups best (area under the receiver operating characteristic curve = 0.80). Intracranial EEG is a key tool to delineate the epileptogenic zone and help render individuals seizure-free postoperatively. We showed that data-driven abnormality maps derived from resting-state magnetoencephalography recordings demonstrate clinical value and may help guide electrode placement in individuals with neocortical epilepsy. Additionally, our predictive model of postoperative seizure freedom, which leverages both magnetoencephalography and intracranial EEG recordings, could aid patient counselling of expected outcome.
Keywords: MEG; epilepsy; iEEG; normative; surgery.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures
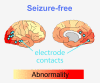

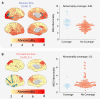


Update of
-
Interictal MEG abnormalities to guide intracranial electrode implantation and predict surgical outcome.ArXiv [Preprint]. 2023 Apr 11:arXiv:2304.05199v1. ArXiv. 2023. Update in: Brain Commun. 2023 Oct 25;5(6):fcad292. doi: 10.1093/braincomms/fcad292. PMID: 37090233 Free PMC article. Updated. Preprint.
References
-
- Kim DW, Kim HK, Lee SK, Chu K, Chung CK. Extent of neocortical resection and surgical outcome of epilepsy: Intracranial EEG analysis. Epilepsia 2010;51(6):1010–1017. - PubMed
-
- Megevand P, Spinelli L, Genetti M, et al. Electric source imaging of interictal activity accurately localises the seizure onset zone. J Neurol Neurosurg Psychiatry. 2014;85(1):38–43. - PubMed
-
- Kim DW, Lee SK, Moon HJ, Jung KY, Chu K, Chung CK. Surgical treatment of nonlesional neocortical epilepsy: Long-term longitudinal study. JAMA Neurol. 2017;74(3):324–331. - PubMed
-
- Foley E, Quitadamo LR, Walsh AR, Bill P, Hillebrand A, Seri S. MEG detection of high frequency oscillations and intracranial-EEG validation in pediatric epilepsy surgery. Clin Neurophysiol. 2021;132(9):2136–2145. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
