Loss of Cadherin-11 in pancreatic ductal adenocarcinoma alters tumor-immune microenvironment
- PMID: 37954069
- PMCID: PMC10639148
- DOI: 10.3389/fonc.2023.1286861
Loss of Cadherin-11 in pancreatic ductal adenocarcinoma alters tumor-immune microenvironment
Abstract
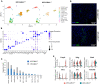
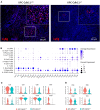
Pancreatic ductal adenocarcinoma (PDAC) is one of the top five deadliest forms of cancer with very few treatment options. The 5-year survival rate for PDAC is 10% following diagnosis. Cadherin 11 (Cdh11), a cell-to-cell adhesion molecule, has been suggested to promote tumor growth and immunosuppression in PDAC, and Cdh11 inhibition significantly extended survival in mice with PDAC. However, the mechanisms by which Cdh11 deficiency influences PDAC progression and anti-tumor immune responses have yet to be fully elucidated. To investigate Cdh11-deficiency induced changes in PDAC tumor microenvironment (TME), we crossed p48-Cre; LSL-KrasG12D/+; LSL-Trp53R172H/+ (KPC) mice with Cdh11+/- mice and performed single-cell RNA sequencing (scRNA-seq) of the non-immune (CD45-) and immune (CD45+) compartment of KPC tumor-bearing Cdh11 proficient (KPC-Cdh11+/+) and Cdh11 deficient (KPC-Cdh11+/-) mice. Our analysis showed that Cdh11 is expressed primarily in cancer-associated fibroblasts (CAFs) and at low levels in epithelial cells undergoing epithelial-to-mesenchymal transition (EMT). Cdh11 deficiency altered the molecular profile of CAFs, leading to a decrease in the expression of myofibroblast markers such as Acta2 and Tagln and cytokines such as Il6, Il33 and Midkine (Mdk). We also observed a significant decrease in the presence of monocytes/macrophages and neutrophils in KPC-Cdh11+/- tumors while the proportion of T cells was increased. Additionally, myeloid lineage cells from Cdh11-deficient tumors had reduced expression of immunosuppressive cytokines that have previously been shown to play a role in immune suppression. In summary, our data suggests that Cdh11 deficiency significantly alters the fibroblast and immune microenvironments and contributes to the reduction of immunosuppressive cytokines, leading to an increase in anti-tumor immunity and enhanced survival.
Keywords: CAF; CDH11; IL33; MDSC; PDAC; T cells.
Copyright © 2023 Sebastian, Martin, Peran, Hum, Leon, Amiri, Wilson, Coleman, Wheeler, Byers and Loots.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

