Efficient cyanide sensing using plasmonic Ag/Fe3O4 nanoparticles
- PMID: 37954410
- PMCID: PMC10633889
- DOI: 10.1039/d3ra06654a
Efficient cyanide sensing using plasmonic Ag/Fe3O4 nanoparticles
Abstract
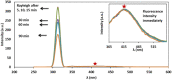
In the line of our previous studies, we have reported a developed sensitive and selective probe for cyanide detection based on Ag/Fe3O4 nanoparticles (NPs) with an extremely low limit of detection at the level of ng per milliliter. Herein, we report the improvement of the easy-to-make magnetic silver nanoparticle-based sensor system for cyanide determination in an extended calibration range with higher selectivity and precision. As far as our knowledge is concerned, the detectable linear range from 1.0 nM to 160 μM (0.026 ng mL-1 to 4.16 μg mL-1) of the improved simple highly precise technique represents the widest assay that has been reported so far. The method is based on strong enhancement of scattered light of the plasmonic nanoparticles and simultaneously cyanide fluorescence quenching. Although the fluorescence of cyanide is highly selective and precise, its intensity is poor. On the other hand, the strongly enhanced Rayleigh signal has a low repeatability. We proposed a method to remove the interference and obtained an effective factor that is directly proportional to cyanide concentration utilizing both above signals simultaneously. In this work, Ag/Fe3O4 NPs have been synthesized easily using a green preparation method and the NPs were consequently characterized using powder XRD, UV-Vis absorption spectroscopy, transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDX). A combination of absorption, Rayleigh and fluorescence characteristics were used for detection of cyanide in real samples and an overview of recently reported sensors for cyanide was also provided.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Panja S. Panja A. Ghosh K. Mater. Chem. Front. 2021;5(2):584–602.
-
- Huang X. Gu X. Zhang G. Zhang D. Chem. Commun. 2012;48(100):12195–12197. - PubMed
-
- Erdemir S. Malkondu S. Talanta. 2020;207:120278. - PubMed
-
- Li J. Yuan S. Qin J. S. Pang J. Zhang P. Zhang Y. Huang Y. Drake H. F. Liu W. R. Zhou H. C. Angew. Chem. 2020;132:9405–9409. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

