Immune Effects of Cryoablation in Woodchuck Hepatocellular Carcinoma
- PMID: 37954494
- PMCID: PMC10637190
- DOI: 10.2147/JHC.S426442
Immune Effects of Cryoablation in Woodchuck Hepatocellular Carcinoma
Abstract
Objectives: Local and systemic immune responses evoked by locoregional therapies such as cryoablation are incompletely understood. The aim of this study was to characterize cryoablation-related immune response and the capacity of immune drugs to augment immunity upon cryoablation for the treatment of hepatocellular carcinoma (HCC) using a woodchuck hepatocellular carcinoma model.
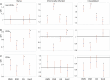
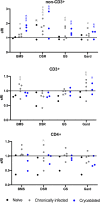
Materials and methods: Twelve woodchucks chronically infected with woodchuck hepatitis virus and with hepatocellular carcinoma underwent imaging with contrast-enhanced CT. Partial cryoablation of tumors in three woodchucks was performed. Fourteen days after cryoablation, liver tissues were harvested and stained with H&E and TUNEL, and immune infiltrates were quantified. Peripheral blood mononuclear cells (PBMC) were collected from ablated and nonablated woodchucks, labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured with immune-modulating drugs, including a small PD-L1 antagonist molecule (BMS-202) and three TLR7/8 agonists (DSR 6434, GS-9620, gardiquimod). After incubation, cell replication and immune cell populations were analyzed by flow cytometry.
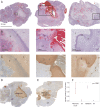
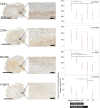
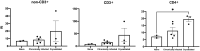
Results: Local immune response in tumors was characterized by an increased number of CD3+ T lymphocytes and natural killer cells in the cryolesion margin compared to other tumor regions. T regulatory cells were found in higher numbers in distant tumors within the liver compared to untreated or control tumors. Cryoablation also augmented the systemic immune response as demonstrated by higher numbers of PBMC responses upon immune drug stimulation in the cryoablation group.
Conclusions: Partial cryoablation augmented immune effects in both treated and remote untreated tumor microenvironments, as well as systemically, in woodchucks with HCC. Characterization of these mechanisms may enhance development of novel drug-device combinations for treatment of HCC.
Keywords: cryoablation; hepatitis B virus; hepatocellular carcinoma; immune effect; tumor microenvironment; woodchuck.
© 2023 Mauda-Havakuk et al.
Conflict of interest statement
Dr Andrew S Mikhail reports grants from BTG (Now Boston Scientific), during the conduct of the study. Dr William F Pritchard reports the NIH and Boston Scientific Corporation (previously Biocompatibles UK Ltd) have a Cooperative Research and Development Agreement providing support for this research, non-financial support from Northeastern Wildlife, during the conduct of the study. Dr Bradford J Wood reports non-financial support from Philips, non-financial support from Boston Scientific / BTG Biocompatibles, during the conduct of the study; non-financial support, Cooperative Research and Development Agreement [CRADA] from Siemens, grants, non-financial support from NVIDIA, grants, Cooperative Research and Development Agreement [CRADA] from Celsion/Immunon, personal fees from Philips, non-financial support, negotiating Cooperative Research and Development Agreement [CRADA]. Negotiating licensing agreement from Canon Medical, non-financial support, Equipment MTA from Medtronic, grants, Cooperative Research and Development Agreement [CRADA] from XAct Robotics, non-financial support, Equipment MTA from Angiodynamics, non-financial support, Equipment MTA from Imactis, non-financial support, Equipment MTA from Profound MEdical, grants, non-financial support, Cooperative Research and Development Agreement [CRADA] from ProMaxo, non-financial support, Equipment MTA from QT Imaging, non-financial support, Supplies MTA from Theromics, non-financial support, Equipment MTA from MediView, non-financial support, Equipment MTA from Johnson and Johnson, non-financial support, Equipment MTA from Clinical Laserthermia Systems, non-financial support, Supplies MTA from Varian, non-financial support, Equipment MTA from Civco Medical, non-financial support, Equipment MTA from Combat Medical, non-financial support, Equipment MTA from Galvanize, outside the submitted work; In addition, Dr Bradford J Wood has a patent portfolio available upon request with royalties paid to see above. The authors report no other conflicts of interest in this work.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

