Various Doses of Tanezumab in the Management of Chronic Low Back Pain (CLBP): A Pooled Analysis of 4,514 Patients
- PMID: 37954824
- PMCID: PMC10634383
- DOI: 10.7759/cureus.46790
Various Doses of Tanezumab in the Management of Chronic Low Back Pain (CLBP): A Pooled Analysis of 4,514 Patients
Abstract
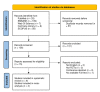
Chronic low back pain (CLBP) is a persistent and debilitating condition characterized by pain and discomfort in the lower back region that lasts more than 12 weeks. This review aims to determine the efficacy and safety of various doses of tanezumab for managing CLBP. The present meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and the Cochrane Handbook for Systematic Reviews of Intervention standards. We searched multiple databases, including PubMed, Cochrane Library, Excerpta Medica Database (EMBASE), Scopus, and Web of Science, to identify randomized controlled trials comparing tanezumab to placebo or different dosage regimens for CLBP in adult patients. The primary outcome was the mean change in low back pain intensity (LBPI) score baseline to the end of treatment. Secondary outcomes included adverse events and the degree of disability or impairment. A total of six studies were included in the meta-analysis. Analysis of the data showed that tanezumab 5 mg significantly reduced LBPI compared to placebo at all time points (mean deviation (MD) ranging from -0.31 to -0.5). Similarly, tanezumab 10 mg showed a significant reduction in LBPI compared to placebo at all time points (MD ranging from -0.48 to -0.84). However, tanezumab 5 mg showed significantly less reduction of LBPI compared to 10 mg at two, four, eight, and 12 weeks (MD ranging from 0.19 to 0.32). These findings suggest that tanezumab is an effective treatment for CLBP, with 5 mg and 10 mg doses providing clinically meaningful reductions in LBPI.
Keywords: chronic low back pain (clbp); chronic pain management; systematic review and meta-analysis; tanezumab; treating low back pain.
Copyright © 2023, Tahir et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




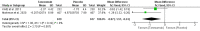
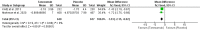






References
-
- Low back pain (chronic) Chou R. http://www.fda.gov/medwatch BMJ Clin Evid. 2010;2010:1116. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
