Pegmolesatide for the treatment of anemia in patients undergoing dialysis: a randomized clinical trial
- PMID: 37954906
- PMCID: PMC10632410
- DOI: 10.1016/j.eclinm.2023.102273
Pegmolesatide for the treatment of anemia in patients undergoing dialysis: a randomized clinical trial
Abstract
Background: Pegmolesatide, a synthetic peptide-based erythropoietin (EPO) receptor agonist, is being evaluated as an alternative to epoetin alfa for treating anemia of chronic kidney disease (CKD) in Chinese dialysis patients. There is a critical need for a long-acting, cost-effective erythropoiesis-stimulating agent that does not produce EPO antibodies.
Methods: A randomized, open-label, active-comparator, non-inferiority phase three trial was conducted at 43 dialysis centers in China between May 17th, 2019, and March 28th, 2022. Eligible patients aged 18-70 years were randomly assigned (2:1) to receive pegmolesatide once every four weeks or epoetin alfa one to three times per week, with doses adjusted to maintain a hemoglobin level between 10.0 and 12.0 g/dL. The primary efficacy endpoint was the mean change in hemoglobin level from baseline to the efficacy evaluation period in the per-protocol set (PPS) population. Non-inferiority of pegmolesatide to epoetin alfa was established if the lower limit of the two-sided 95% confidence interval for the between-group difference was ≥ -1.0 g/dL. Safety assessment included adverse events and potential anaphylaxis reactions. This trial is registered at ClinicalTrials.gov, NCT03902691.
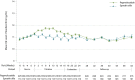
Findings: Three hundreds and seventy-two patients were randomly assigned to the pegmolesatide group (248 patients) or the epoetin alfa group (124 patients). A total of 347 patients (233 in the pegmolesatide group and 114 in the epoetin alfa group) were included in the PPS population. In the PPS, the mean change (standard deviation, SD) in hemoglobin level from baseline to the efficacy evaluation period was 0.07 (0.92) g/dL in the pegmolesatide group and -0.22 (0.97) g/dL in the epoetin alfa group. The between-group difference was 0.29 g/dL (95% confidence interval: 0.11-0.47), verifying non-inferiority of pegmolesatide to epoetin alfa. Adverse events occurred in 231 (94%) participants in the pegmolesatide group and in 110 (89%) in the epoetin alfa group. Hypertension was the most common treatment-related adverse event. No fatal cases of anaphylaxis or hypotension were reported.
Interpretation: Monthly subcutaneously injection of pegmolesatide was as effective and safe as conventional epoetin alfa administrated one to three times a week in treating anemia in Chinese dialysis patients.
Funding: The study was supported by Hansoh Medical Development Group.
Keywords: Anemia; Chronic kidney disease; Dialysis; Epoetin alfa; Pegmolesatide.
© 2023 The Authors.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures
References
-
- McMurray J., Parfrey P., Adamson J., et al. Kidney disease: improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335.
-
- Working Group on Renal Anemia Guidelines NB CPA Clinical practice guidelines for diagnosis and treatment of renal anemia in China. Zhonghua Yixue Zazhi. 2021;101:1463–1502.
-
- Eschbach J.W., Egrie J.C., Downing M.R., Browne J.K., Adamson J.W. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–78. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials