Disease activity 4.5 years after starting cladribine: experience in 264 patients with multiple sclerosis
- PMID: 37954917
- PMCID: PMC10638874
- DOI: 10.1177/17562864231200627
Disease activity 4.5 years after starting cladribine: experience in 264 patients with multiple sclerosis
Abstract
Background: Cladribine is an effective immunotherapy for people with multiple sclerosis (pwMS). Whilst most pwMS do not require re-treatment following standard dosing (two treatment courses), disease activity re-emerges in others. The characteristics of pwMS developing re-emerging disease activity remain incompletely understood.
Objectives: To explore whether clinical and/or paraclinical baseline characteristics, including the degree of lymphocyte reduction, drug dose and lesions on magnetic resonance imaging (MRI) are associated with re-emerging disease activity.
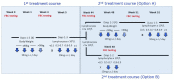
Design: Service evaluation in pwMS undergoing subcutaneous cladribine (SClad) treatment.
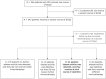
Methods: Demographics, clinical, laboratory and MRI data of pwMS receiving two courses of SClad were extracted from health records. To assess associations of predictor variables with re-emerging disease activity, a series of Cox proportional hazards models was fitted (one for each predictor variable).
Results: Of n = 264 pwMS 236 received two courses of SClad and were included in the analysis. Median follow-up was 4.5 years (3.9, 5.3) from the first, and 3.5 years (2.9, 4.3) from the last SClad administration. Re-emerging disease activity occurred in 57/236 pwMS (24%); 22/236 received further cladribine doses (SClad or cladribine tablets) at 36.7 months [median; interquartile range (IQR): 31.7, 42.1], and 22/236 other immunotherapies 18.9 months (13.0, 30.2) after their second course of SClad, respectively. Eligibility was based on MRI activity in 29, relapse in 5, both in 13, elevated cerebrospinal fluid neurofilament light chain level in 3, deterioration unrelated to relapse in 4 and other in 3. Only 36/57 of those eligible for additional immunotherapy had received a reduced dose of SClad for their second treatment course. Association was detected between re-emerging disease activity and (i) high baseline MRI activity and (ii) low second dose of SClad.
Conclusion: Re-emerging disease activity was associated with baseline MRI activity and low dose second course of SClad.
Keywords: cladribine; disease activity; disease breakthrough; multiple sclerosis; re-treatment; treatment switch.
© The Author(s), 2023.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KA-P, SDT, AMD, AA, LB, TC and DWH have no conflicts of interest to declare. GG has received honoraria and meeting support from AbbVie Biotherapeutics, Biogen, Canbex, Ironwood, Novartis, Merck, Merck Serono, Roche, Sanofi Genzyme, Synthon, Teva and Vertex. He also serves as a chief editor for Multiple Sclerosis and Related Disorders. SG has received honoraria from Biogen Idec, Sanofi Genzyme, Janssen Cilag, Merck, Neurodiem, Novartis, Roche and Teva, and grant support from ECTRIMS, Genzyme, Merck, National MS Society, Takeda, UK MS Society, NIHR and NHSx. MM has received travel support and speaker honoraria from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche and Teva, and consultation for Celgene, Merck Serono, Novartis and Roche. JM has received honoraria and meeting support from Arvelle, Biogen, Novartis, Merck Serono, Roche, Sanofi Genzyme, Jazz Pharmaceuticals, Janssen and Novartis. BPT has received honoraria, travel grants, and has been a member of advisory boards for Biogen, Merck Serono, Novartis, Sanofi Genzyme and Roche. DB has received compensation from InMuneBio, Lundbeck, Merck, Novartis, Rock and Teva. KS has received research support, through Queen Mary University of London, from Biogen, Merck KGaA and Novartis, speaking honoraria from, and/or served in an advisory role for, Biogen, EMD Serono, Merck KGaA, Novartis, Roche, Sanofi-Genzyme and Teva; and remuneration for teaching activities from AcadeMe, Medscape and the Neurology Academy.
Figures

References
-
- Giovannoni G, Comi G, Cook S, et al.. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. - PubMed
-
- Baker D, Pryce G, Herrod SS, et al.. Potential mechanisms of action related to the efficacy and safety of cladribine. Mult Scler Relat Disord 2019; 30: 176–186. - PubMed
LinkOut - more resources
Full Text Sources

