Breathing and Oxygen Carrying Capacity in Ts65Dn and Down Syndrome
- PMID: 37954975
- PMCID: PMC10634617
- DOI: 10.1093/function/zqad058
Breathing and Oxygen Carrying Capacity in Ts65Dn and Down Syndrome
Abstract
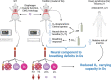
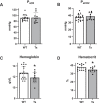
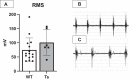
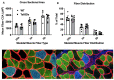
Individuals with Down syndrome (Ds) are at increased risk of respiratory infection, aspiration pneumonia, and apnea. The Ts65Dn mouse is a commonly used model of Ds, but there have been no formal investigations of awake breathing and respiratory muscle function in these mice. We hypothesized that breathing would be impaired in Ts65Dn vs. wild-type (WT), and would be mediated by both neural and muscular inputs. Baseline minute ventilation was not different at 3, 6, or 12 mo of age. However, VT/Ti, a marker of the neural drive to breathe, was lower in Ts65Dn vs. WT and central apneas were more prevalent. The response to breathing hypoxia was not different, but the response to hypercapnia was attenuated, revealing a difference in carbon dioxide sensing, and/or motor output in Ts65Dn. Oxygen desaturations were present in room air, demonstrating that ventilation may not be sufficient to maintain adequate oxygen saturation in Ts65Dn. We observed no differences in arterial PO2 or PCO2, but Ts65Dn had lower hemoglobin and hematocrit. A retrospective medical record review of 52,346 Ds and 52,346 controls confirmed an elevated relative risk of anemia in Ds. We also performed eupneic in-vivo electromyography and in-vitro muscle function and histological fiber typing of the diaphragm, and found no difference between strains. Overall, conscious respiration is impaired in Ts65Dn, is mediated by neural mechanisms, and results in reduced hemoglobin saturation. Oxygen carrying capacity is reduced in Ts65Dn vs. WT, and we demonstrate that individuals with Ds are also at increased risk of anemia.
Keywords: apnea; diaphragm; hypercapnia; hypoxemia; hypoxia; plethysmography; trisomy 21; ventilation.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
None of the authors has any conflict of interest.
Figures




References
-
- Egan JF, Benn PA, Zelop CM, Bolnick A, Gianferrari E, Borgida AF. Down syndrome births in the United States from 1989 to 2001. Am J Obstet Gynecol. 2004;191(3):1044–1048. - PubMed
-
- Shin M, Besser LM, Kucik JEet al. Prevalence of Down syndrome among children and adolescents in 10 regions of the United States. Pediatrics. 2009;124(6):1565–1571. - PubMed
-
- Birth defects surveillance data from selected states, 1998–2002. Birth Defect Res A. 2005;73(10):758–853. - PubMed
-
- Survey of Income and Program Participation . 2006.
-
- Lane SBaDL, ed. The Development of Intelligence. New York, NY: Holt, Rinehart and Winston, 1985.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
