Expression Profiles of circRNAs and Identification of hsa_circ_0007608 and hsa_circ_0064656 as Potential Biomarkers for COPD-PH Patients
- PMID: 37955024
- PMCID: PMC10638933
- DOI: 10.2147/COPD.S424712
Expression Profiles of circRNAs and Identification of hsa_circ_0007608 and hsa_circ_0064656 as Potential Biomarkers for COPD-PH Patients
Abstract
Introduction: Pulmonary hypertension (PH) is a common complication of chronic obstructive pulmonary disease (COPD), which can worsen the prognosis and increase the mortality of COPD patients. Circular RNA (circRNA) has been discovered to participate in the occurrence and progression of PH in COPD and may have significant prospects for advanced diagnostics and prognosis evaluation. However, the expression profile of circRNAs in human lung tissues with definite diagnosis of COPD-PH remains to be further explored and validated.
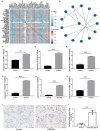
Methods: Twelve human lung tissue samples (6 each from COPD-PH and control groups) were collected and subjected to high-throughput sequencing. QRT-PCR was performed to validate the differential expression levels of the top 10 dysregulated circRNAs in patients' plasma samples, HPAECs and HPASMCs. Functional and pathway enrichment analysis on target genes was performed to explore the potential functions and pathways of those circRNAs. Hub genes obtained after conducting bioinformatics analysis on the predicted target mRNAs were verified by qRT-PCR in HPAECs and HPASMCs, and then we selected VCAN as a potential key gene involved in the pathogenesis of COPD-PH for immunohistochemistry validation in lung tissue.
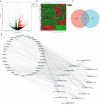
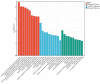
Results: A total of 136 circRNAs (39 up-regulated and 97 down-regulated) were differentially expressed between the two groups. Following qRT-PCR validation, two circRNAs (hsa_circ_0007608 and hsa_circ_0064656) were believed to be involved in the pathogenesis. GO and KEGG pathway analysis suggested that these two DECs were mainly related to the celluar proliferation, migration and EndMT. PPI network revealed 11 pairs of key mRNAs. VCAM1, VCAN and THBS1, three hub mRNAs with the highest reliability among all, were validated and proven to be up-regulated in COPD-PH. We innovatively found that VCAN may be involved in COPD-PH.
Conclusion: This study identified the functional circRNAs, providing insights into the molecular mechanisms and predictions of COPD-PH, and may provide potential diagnostic biomarkers or therapeutic targets for COPD-PH.
Keywords: RNA-sequencing; bioinformatics analysis; biomarker; chronic obstructive pulmonary disease; circular RNA; pulmonary hypertension.
© 2023 Yu et al.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures




Similar articles
-
Identification of circRNA-associated ceRNA networks in peripheral blood mononuclear cells as potential biomarkers for chronic obstructive pulmonary disease.Biosci Rep. 2023 Oct 31;43(10):BSR20230005. doi: 10.1042/BSR20230005. Biosci Rep. 2023. PMID: 37650285 Free PMC article.
-
A comprehensive evaluation of skin aging-related circular RNA expression profiles.J Clin Lab Anal. 2021 Apr;35(4):e23714. doi: 10.1002/jcla.23714. Epub 2021 Feb 3. J Clin Lab Anal. 2021. PMID: 33534927 Free PMC article.
-
Identification and bioinformatic analysis of CircRNAs in the plasma of patients with very severe chronic obstructive pulmonary disease.BMC Pulm Med. 2023 Jun 16;23(1):211. doi: 10.1186/s12890-023-02513-5. BMC Pulm Med. 2023. PMID: 37328740 Free PMC article.
-
Exploring the association between circRNA expression and pediatric obesity based on a case-control study and related bioinformatics analysis.BMC Pediatr. 2023 Nov 13;23(1):561. doi: 10.1186/s12887-023-04261-1. BMC Pediatr. 2023. PMID: 37957626 Free PMC article. Review.
-
Exploring circular RNAs as biomarkers for Parkinson's disease and their expression changes after aerobic exercise rehabilitation.Funct Integr Genomics. 2024 Jul 29;24(4):130. doi: 10.1007/s10142-024-01409-9. Funct Integr Genomics. 2024. PMID: 39069524 Review.
Cited by
-
Promotor Hypomethylation Mediated Upregulation of VCAN Targets Twist1 to Promote EndMT in Hypoxia-Induced Pulmonary Hypertension.J Am Heart Assoc. 2024 Dec 3;13(23):e036969. doi: 10.1161/JAHA.124.036969. Epub 2024 Nov 22. J Am Heart Assoc. 2024. PMID: 39578365 Free PMC article.
-
Circulating Biomarkers in Pulmonary Arterial Hypertension: An Update.Biomolecules. 2024 May 3;14(5):552. doi: 10.3390/biom14050552. Biomolecules. 2024. PMID: 38785959 Free PMC article. Review.
References
-
- Vizza CD, Hoeper MM, Huscher D, et al. Pulmonary Hypertension in Patients With COPD: results From the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Chest. 2021;160(2):678–689. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

